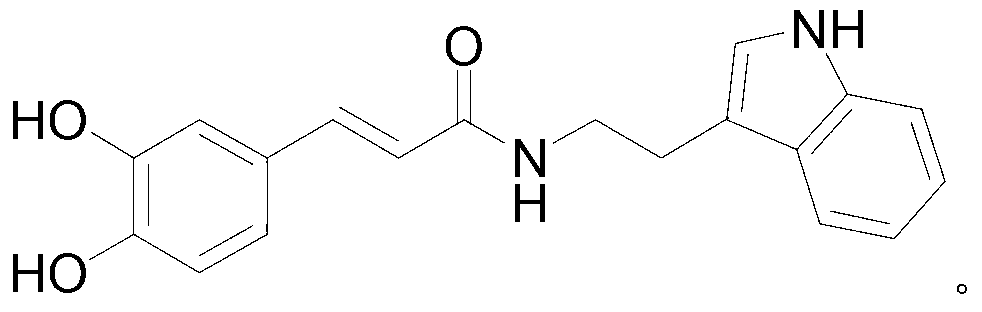
Method for preparing N-coffee acyl tryptamine by one-step process
A technology of acyltryptamine and coffee, applied in the field of drug synthesis, can solve the problems of being unsuitable for large-scale industrial production and long reaction steps, and achieve the effects of facilitating large-scale industrial production, mild reaction conditions, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
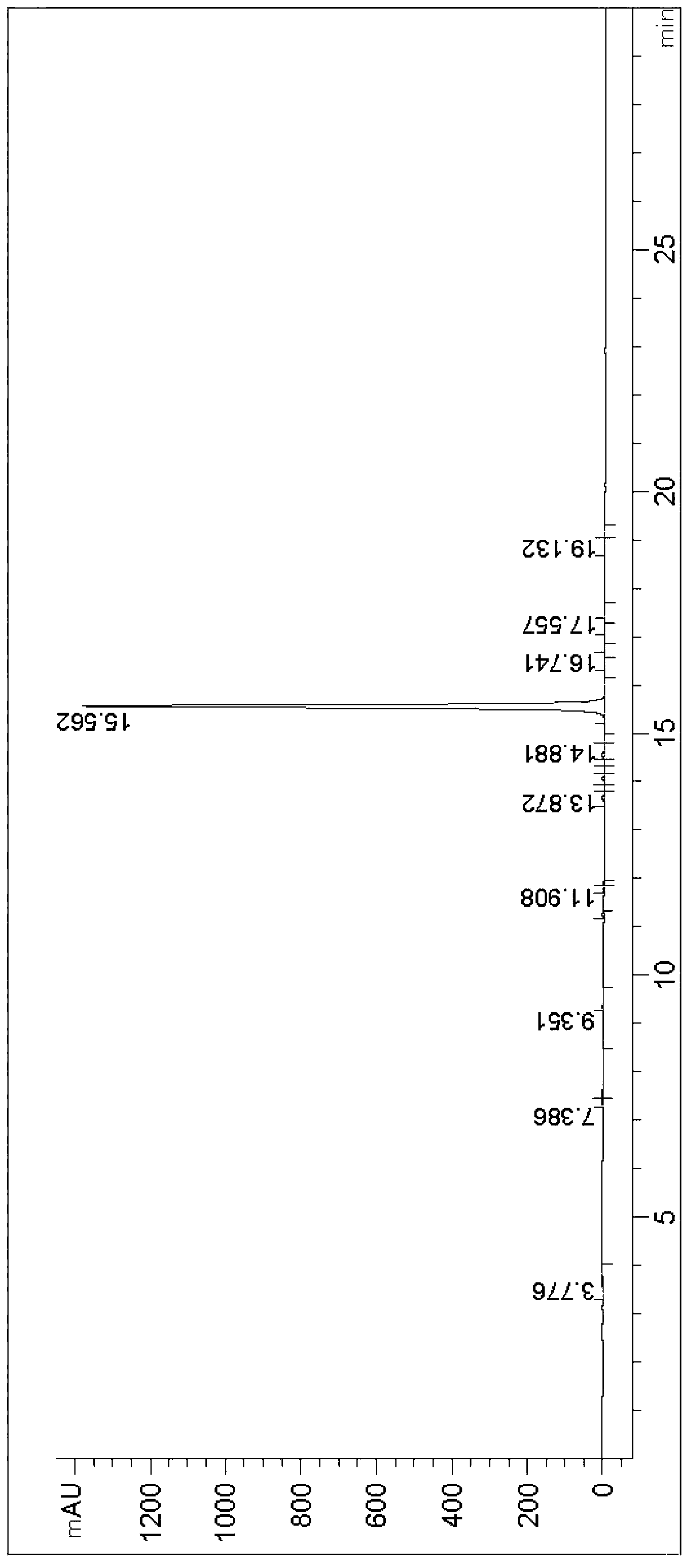
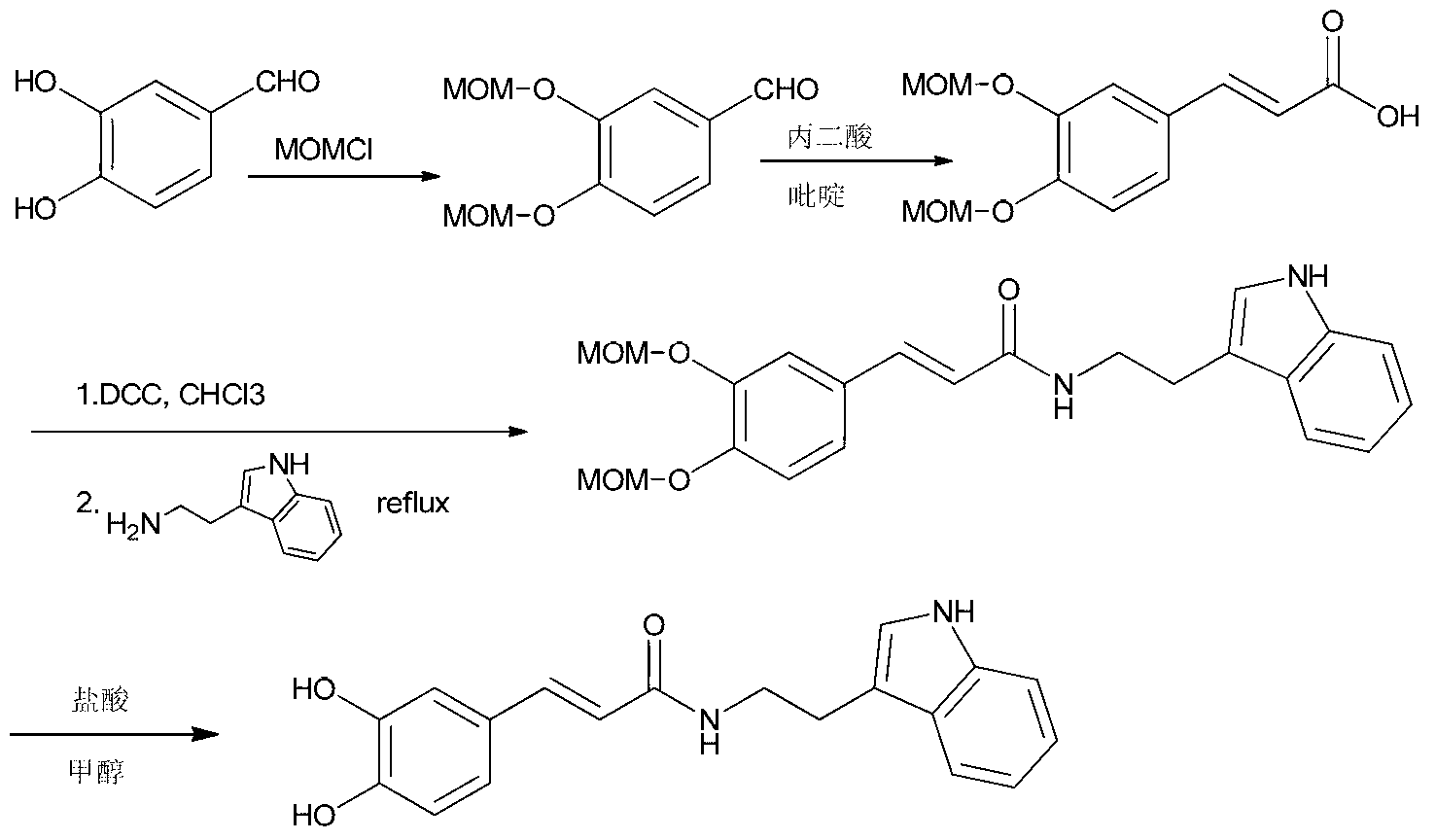
[0033]Add 10g of caffeic acid and 8.89g of tryptamine into a three-necked reaction flask and dissolve in 100mL of N,N-dimethylformamide, and dissolve all of the caffeic acid and tryptamine under stirring to form a reaction solution. Add 15g of 1-hydroxybenzotriazole and 21.1g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride into the reaction solution, after the addition, cool the reaction solution to 0°C, and then 14.3gN,N-diisopropylethylamine was added dropwise to the reaction solution. After the addition, the temperature of the reaction solution was raised to 30° C. for 10 hours until the reaction was complete. Concentrate the reaction solution to remove N,N-dimethylformamide, add 200mL of ethyl acetate and 100mL of 1mol / L hydrochloric acid aqueous solution to the residue, stir for 0.5 hours, let stand to separate layers, separate the upper organic phase and wash with 100mL of saturated carbonic acid Wash once with sodium hydrogen aqueous solution, dry with a...
Embodiment 2
[0035] Add 18g of caffeic acid and 16g of tryptamine into a three-neck reaction flask and dissolve in 200mL of N,N-dimethylacetamide, and dissolve all of the caffeic acid and tryptamine under stirring to form a reaction solution. Add 67.2g of diisopropylcarbodiimide and 23g of N-hydroxysuccinimide into the reaction solution, after the addition, cool the reaction solution to -2°C, then add 20.2g of triethylamine dropwise into the reaction solution . After the addition, the temperature of the reaction solution was raised to 25° C. for 8 hours until the reaction was complete. Concentrate the reaction solution to remove N,N-dimethylacetamide, add 400mL of isopropyl acetate and 200mL of 1mol / L sulfuric acid aqueous solution to the residue, stir for 0.8 hours and then separate the layers, separate the upper organic phase and use 200mL saturated Wash once with aqueous sodium bicarbonate solution, dry with anhydrous sodium sulfate, filter to obtain the filtrate, and concentrate the f...
Embodiment 3
[0037] Add 7.2g of caffeic acid and 6.4g of tryptamine into a three-necked reaction flask and dissolve in 80mL of N-methylpyrrolidone, and stir to dissolve all of the caffeic acid and tryptamine to form a reaction solution. Add 16.5g of dicyclohexylcarbodiimide and 10.9g of 1-hydroxy-7-azobenzotriazole to the reaction solution, after the addition, cool the reaction solution to 2°C, then add 6.3g of pyridine dropwise to the reaction solution in the liquid. After the addition, the temperature of the reaction solution was raised to 35° C. for 12 hours until the reaction was complete. Concentrate the reaction solution to remove N-methylpyrrolidone, add 150mL of dichloromethane and 80mL of 1mol / L formic acid aqueous solution to the residue, stir for 0.5 hours, let stand to separate layers, separate the upper organic phase and wash with 80mL of saturated sodium bicarbonate aqueous solution Once, dry with anhydrous sodium sulfate, filter to obtain the filtrate, concentrate the filtr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com