Trimeric indole compound and its preparation method and use
A compound and catalyst technology, applied in the field of solar cell materials, can solve the problems of difficult purification of conjugated polymers and poor device repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The preparation method of trimeric indole compounds
[0090] Trimeric indole compounds of the present invention can be prepared by conventional synthetic methods known to those skilled in the art. In a class of preferred embodiments of the present invention, the preparation method of the compound comprises the step of: subjecting the compound of formula IV to a boron esterification reaction in the presence of a catalyst, thereby forming the compound of formula V.
[0091]
[0092] (2) In the presence of a catalyst, the compound of formula V is subjected to Suzuki coupling reaction with the compound of formula VI to form the compound of formula I.
[0093]
[0094] In the formula, the definitions of R and X are the same as above; L is I or Br.
[0095] The catalyst can be a conventional catalyst used in the coupling reaction, preferably a palladium catalyst or a combination of a palladium catalyst and a ligand.
[0096] Palladium catalysts include but are not lim...
Embodiment 5
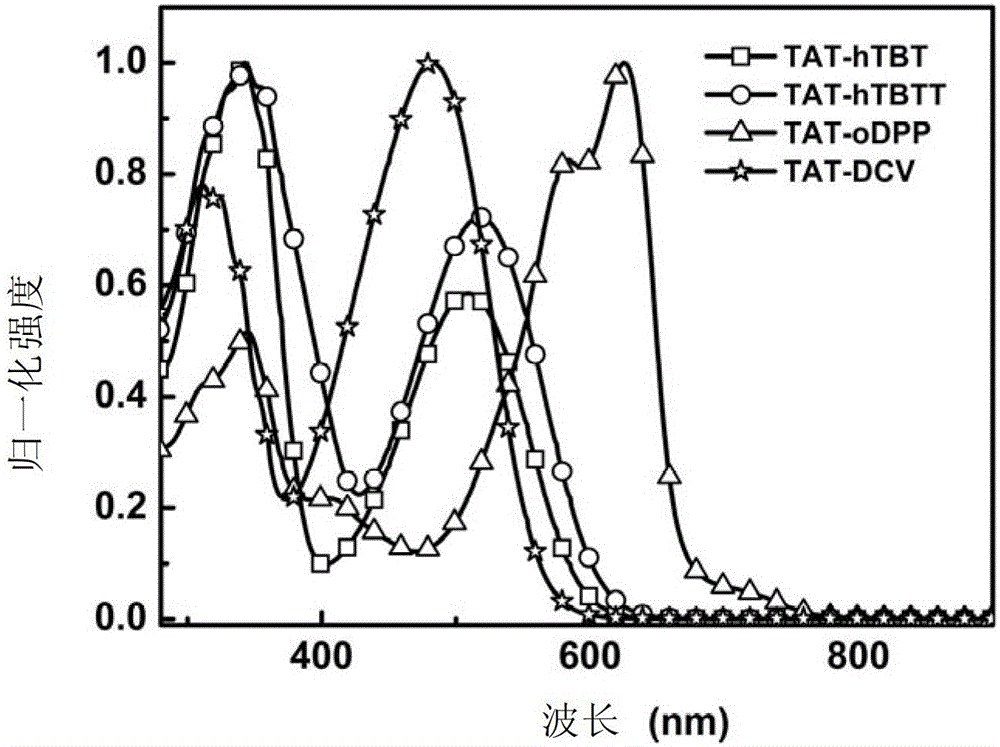
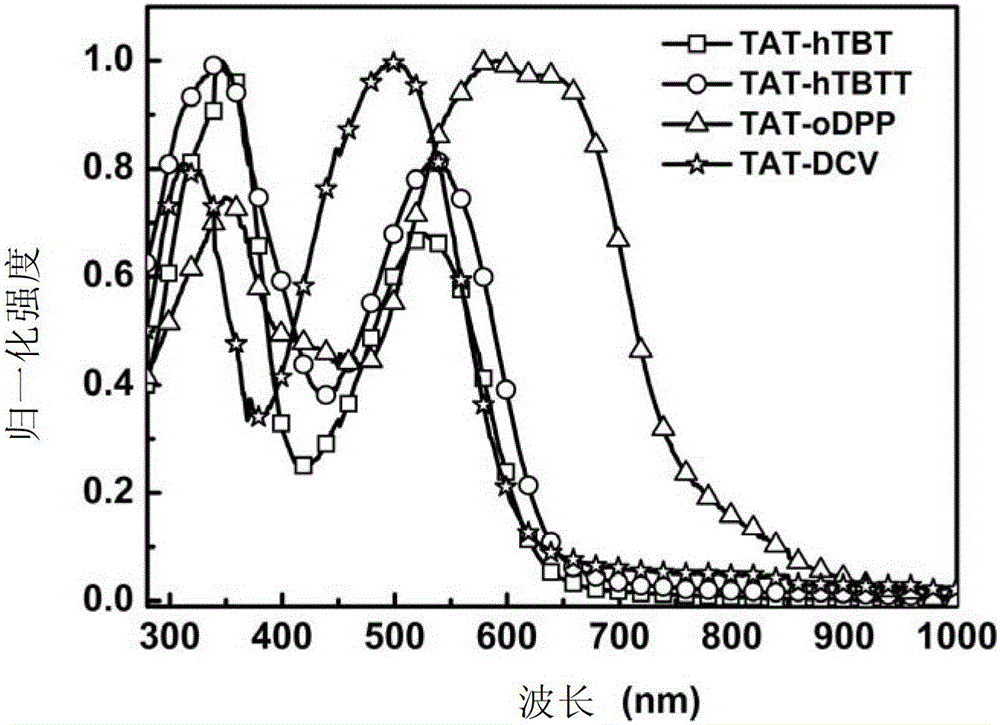
[0263] Embodiment 5: UV-visible absorption spectrum test
[0264] UV-Vis absorption spectrum test of compounds TAT-hTBT, TAT-hTBTT, TAT-oDPP and TAT-DCV in chlorobenzene solution:
[0265] The ultraviolet-visible absorption spectrum of above-mentioned compound is carried out on the ultraviolet-visible spectrophotometer, wherein the used solvent of solution absorption spectrum is chlorobenzene, and concentration is 10 -5 mol / L; Take 2mL of the solution of each compound and add it to a quartz cuvette, put it into a mapadaUV-3300 UV-Vis spectrophotometer, after calibrating the baseline and blank, test the absorption spectrum of the solution in the range of 200-1000nm . The test results show (such as figure 1 As shown), the compound solution described in this application has good absorption in the visible light region, which meets the basic light absorption requirements of organic solar cell donor materials. At the same time, the absorption spectrum of the compound solution is ...
Embodiment 6
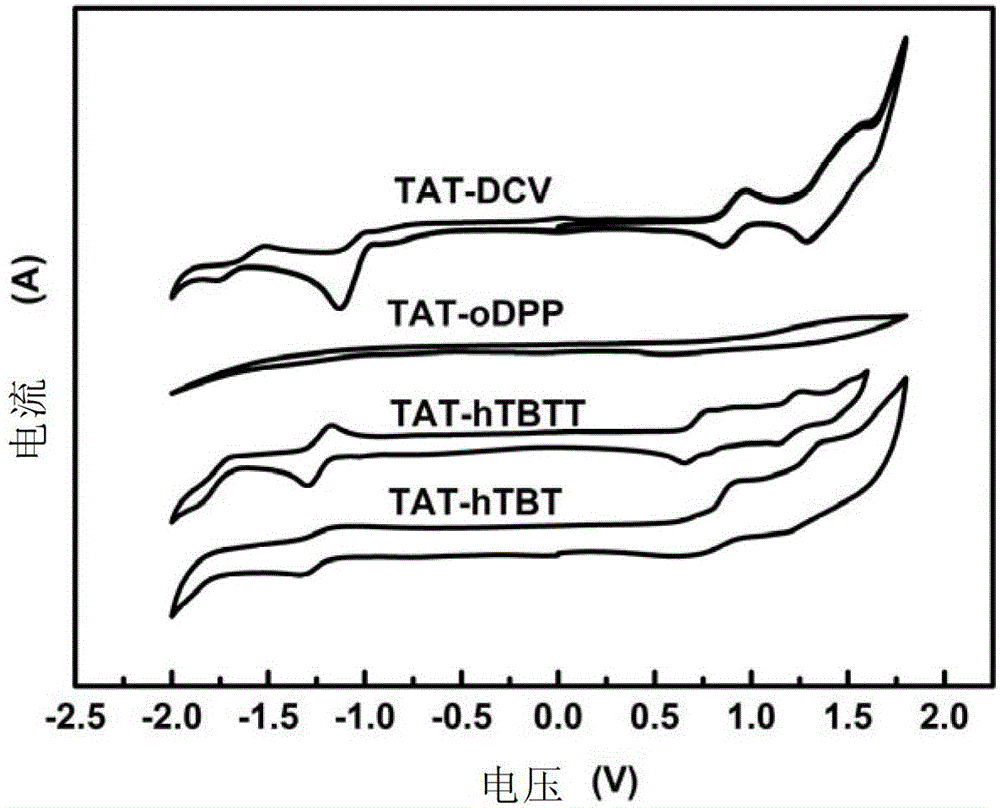
[0268] Embodiment 6: Cyclic voltammetry test
[0269] Cyclic voltammetry tests on compounds TAT-hTBT, TAT-hTBTT, TAT-oDPP and TAT-DCV:
[0270] The energy level of the highest occupied orbital (HOMO) and the lowest unoccupied orbital (LUMO) of the molecule can be calculated by measuring the redox potential of the molecule by cyclic voltammetry. In this embodiment, the electrochemical workstation is used to test the electrochemical properties. The electrolytic cell is a three-electrode system (the glassy carbon electrode is the working electrode, the glass wire electrode is the auxiliary electrode, and the silver / silver chloride electrode is the reference electrode). Ferrocene was used as internal standard, dried dichloromethane or acetonitrile was used as solvent, 0.1mol / L tetrabutylammonium hexafluorophosphate was used as supporting electrolyte, and the scanning speed was 50mV / S. Under argon protection, scan to obtain cyclic voltammetry curve (such as image 3 shown), calcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com