Preparation method and applications of catalyst used for preparing light olefin from synthesis gas
A low-carbon olefin and catalyst technology, which is applied in the field of supported solid iron-based catalyst preparation, can solve the problems of high catalyst preparation cost, high preparation cost and high methane selectivity, and achieves easy large-scale production, mature industrial base, and catalyst activity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
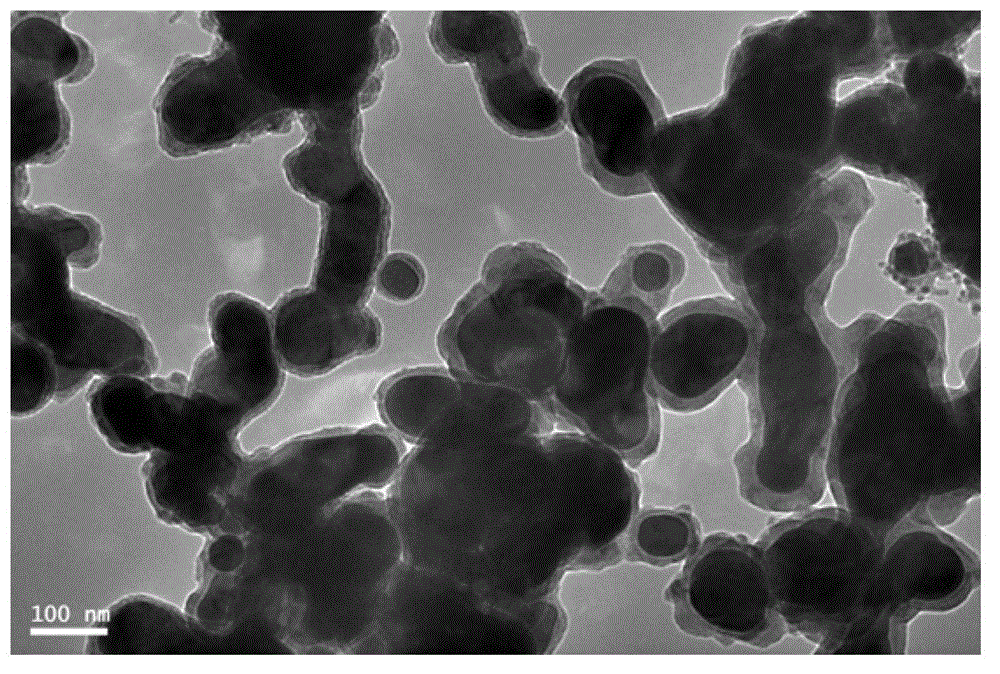
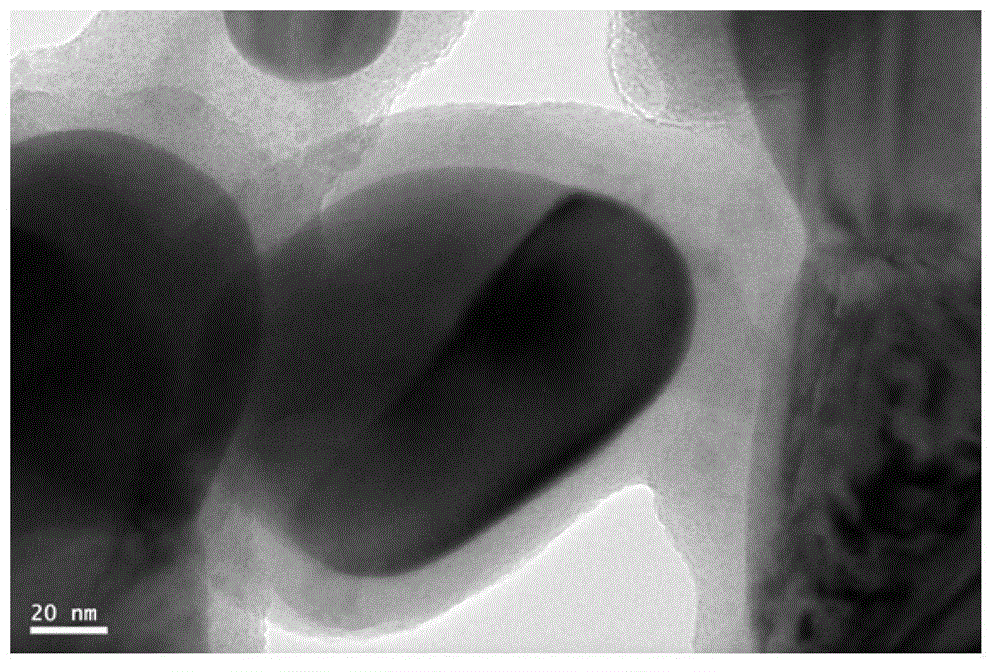
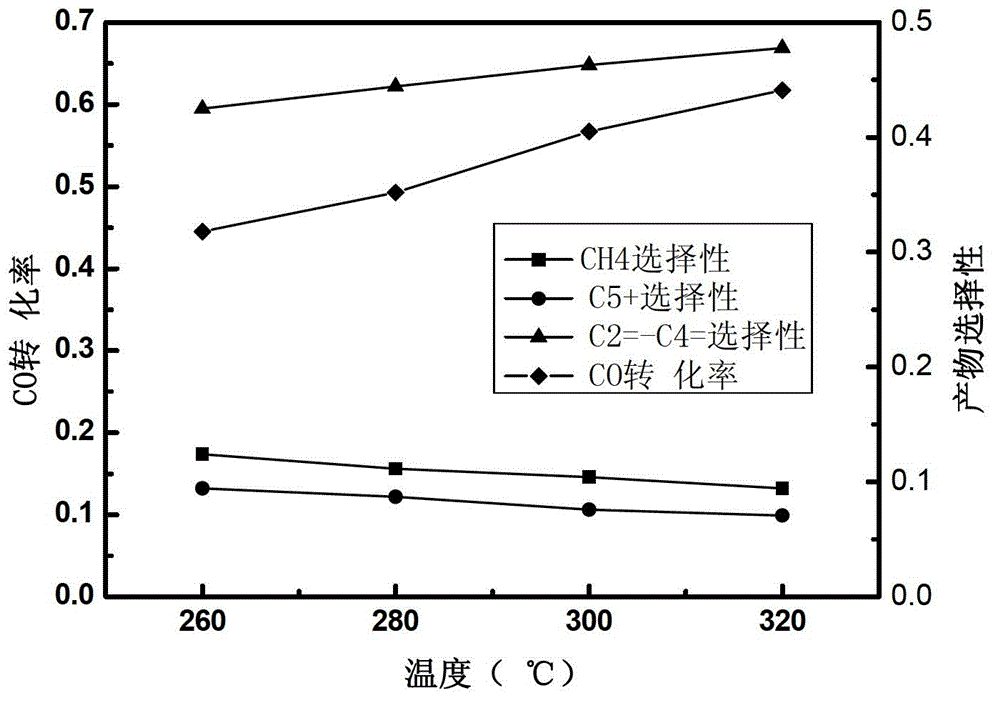
Image
Examples
Embodiment 1
[0041] Embodiment 1: 0.7485g Mn (NO 3 ) 2 .4H 2 O, 0.06510g KNO 3 , 1.0880g Co(NO 3 ) 2 .6H 2 O, 17.57402g Fe(NO 3 ) 3 .9H 2 O was dissolved in 5.75g ethanol, stirred and dissolved until the source solution was uniform. The obtained mixture is blown into the constant temperature section (300-2000° C.) of the tube furnace with a certain flow rate (30-100 L / h) of inert gas and the source solution pumped in by a peristaltic pump at a certain flow rate (20-100 ml / h). The outlet product gas is passed into the cooler for deposition and collection, and the TiO 2 Grind it to 80-200 mesh, take 3g and mix it fully with the sediment, and calcinate at 400°C for 4 hours to obtain a catalyst for preparing low-carbon olefins from the supported direct conversion of synthesis gas.
Embodiment 2
[0042] Embodiment 2: 0.7485g Mn (NO 3 ) 2 .4H 2 O, 0.06510g KNO 3 , 1.0880g Co(NO 3 ) 2 .6H 2 O, 12.1800.1g of ferric ammonium citrate was dissolved in 5.75g of ethanol, stirred and dissolved until the source solution was uniform. Under vacuum, the iron source solution was sonicated. The obtained mixture is blown into the constant temperature section (300-2000° C.) of the tube furnace with a certain flow rate (30-100 L / h) of inert gas and the source solution pumped in by a peristaltic pump at a certain flow rate (20-100 ml / h). The outlet product gas is passed into the cooler for deposition and collection, and the SiO 2 Grind it to 80-200 mesh, take 3g and mix it fully with the sediment, and calcinate at 400°C for 4 hours to obtain a catalyst for preparing low-carbon olefins from the supported direct conversion of synthesis gas.
Embodiment 3
[0043] Embodiment 3: 0.7485g Mn (NO 3 ) 2 .4H 2 O, 0.06510g KNO 3 , 1.0880g Co(NO 3 ) 2 .6H 2 O, 17.57402g Fe(NO3)3.9H2O was dissolved in 4.83g acetone, stirred and dissolved until the source solution was uniform. Under vacuum, the iron source solution was sonicated. The obtained mixture is blown into the constant temperature section (300-2000° C.) of the tube furnace with a certain flow rate (30-100 L / h) of inert gas and the source solution pumped in by a peristaltic pump at a certain flow rate (20-100 ml / h). The outlet product gas is passed into the cooler for deposition and collection, and the Al 2 o 3 Grind it to 80-200 mesh, take 3g and mix it fully with the sediment, and calcinate at 400°C for 4 hours to obtain a catalyst for preparing low-carbon olefins from the supported direct conversion of synthesis gas.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com