Method for implementing constitutive expression of plectasin derivative MP1102 in Pichia pastoris
A technology of MP1102 and plectasin, which is applied in the field of genetic engineering, can solve the problems of MP1102 that have not been seen yet, and achieve the effect of broad application value and market prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
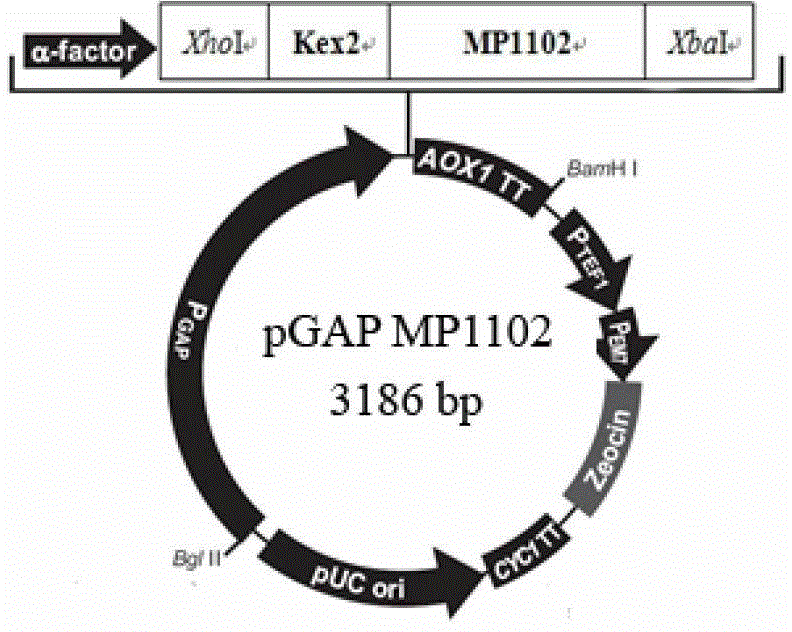
[0077] Example 1 Construction of Pichia pastoris constitutive expression vector pGAPMP1102
[0078] (1) Design and synthesize the following gene fragments: According to the glyceraldehyde 3'-phosphate dehydrogenase promoter (GAP) sequence provided by Invitrogen, the restriction site BglII was added to the 5' end of the sequence, and the 3' end was sequentially added α-factor secretion signal peptide sequence and XhoI restriction site, and the designed gene sequence was sent to Shanghai Sangon Bioengineering Co., Ltd. for synthesis. The nucleotide sequence of the synthesized gene fragment is shown in SEQ ID No.4, GAP promoter The nucleotide sequence of the sequence is shown in SEQ ID No.3;
[0079] (2) The synthetic gene fragment and the vector pUC57 were double-digested with restriction endonucleases BglII and XhoI respectively, and the pUC57 vector fragment and the GAP promoter + α-factor secretion signal peptide fragment were recovered and ligated to obtain the vector pUCG...
Embodiment 2
[0104] Example 2 Construction of recombinant yeast strain containing pGAPMP1102
[0105] 2.1 Linearization of recombinant vector pGAPMP1102
[0106] Use AvrII to digest the constitutive recombinant expression vector pGAPMP1102. The enzyme digestion system and reaction conditions are as follows:
[0107]
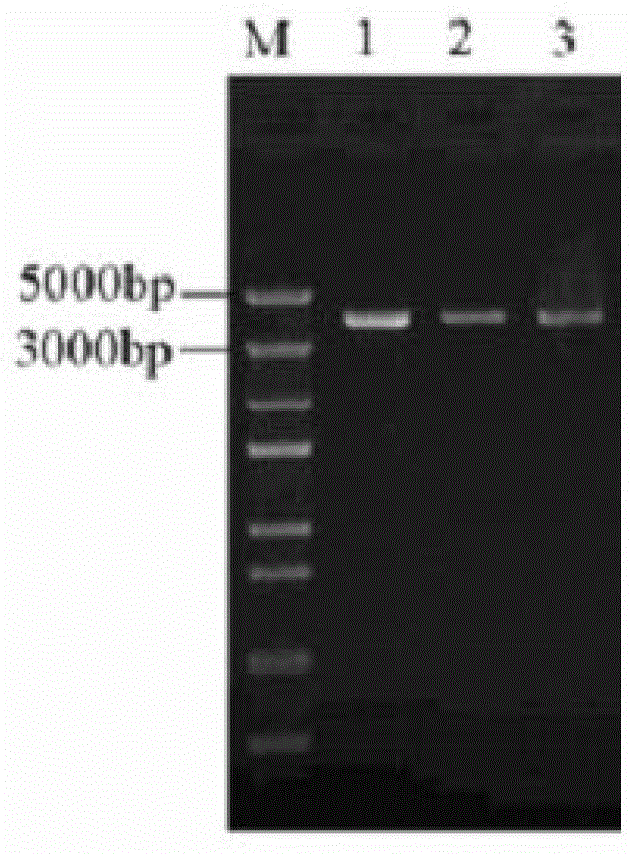
[0108] After adding the above enzyme digestion system, react at 37°C for 3 hours, and detect by 2% agarose gel electrophoresis, electrophoresis conditions: 120 V, 30 min. After the electrophoresis was completed, the linearized recombinant expression vector pGAPMP1102 was recovered using a DNA recovery kit to detect the correct linearization of the recombinant expression vector.
[0109] The results of electrophoresis showed that the linearization effect of the recombinant vector was good ( image 3), there is almost no uncut vector and asterisk activity, and the size is between 5000 bp and 3000 bp, which is consistent with the theoretical size (3186 bp).
[0110] 2.2 Pi...
Embodiment 3
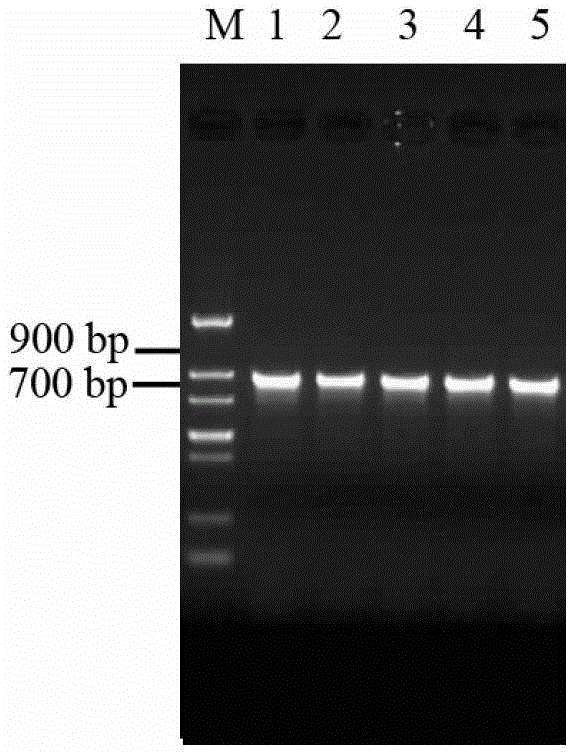
[0128] The results showed that the positive clone rate of transformants was high, and the recombinant yeast PCR band size was 700-900bp ( Figure 4 ), which are consistent with the theoretical size (879bp), indicating that the target gene has been integrated into the yeast genome, and the recombinant yeast Pichia pastorisX-33 / GAPMP02 was obtained. Example 3 Shake Flask Level Constitutive Expression MP1102 Recombinant Yeast Strain Screening
[0129] 3.1 Constitutive expression of transformants at shake flask level
[0130] Pick positive transformants and inoculate them into YPD liquid medium, 30°C, 250rpm shaking culture for 18-20h, transfer 0.5-1% of the inoculum to 50mL YPD liquid medium, 30°C, 250rpm shaking culture for 1 day, then in four layers Sterilized gauze was replaced with cellophane sealing film to wrap the mouth of the shaker flask, and cultured with shaking at 30°C and 250rpm for 3-5 days until the end of fermentation.
[0131] 3.2 Detection of antibacterial act...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com