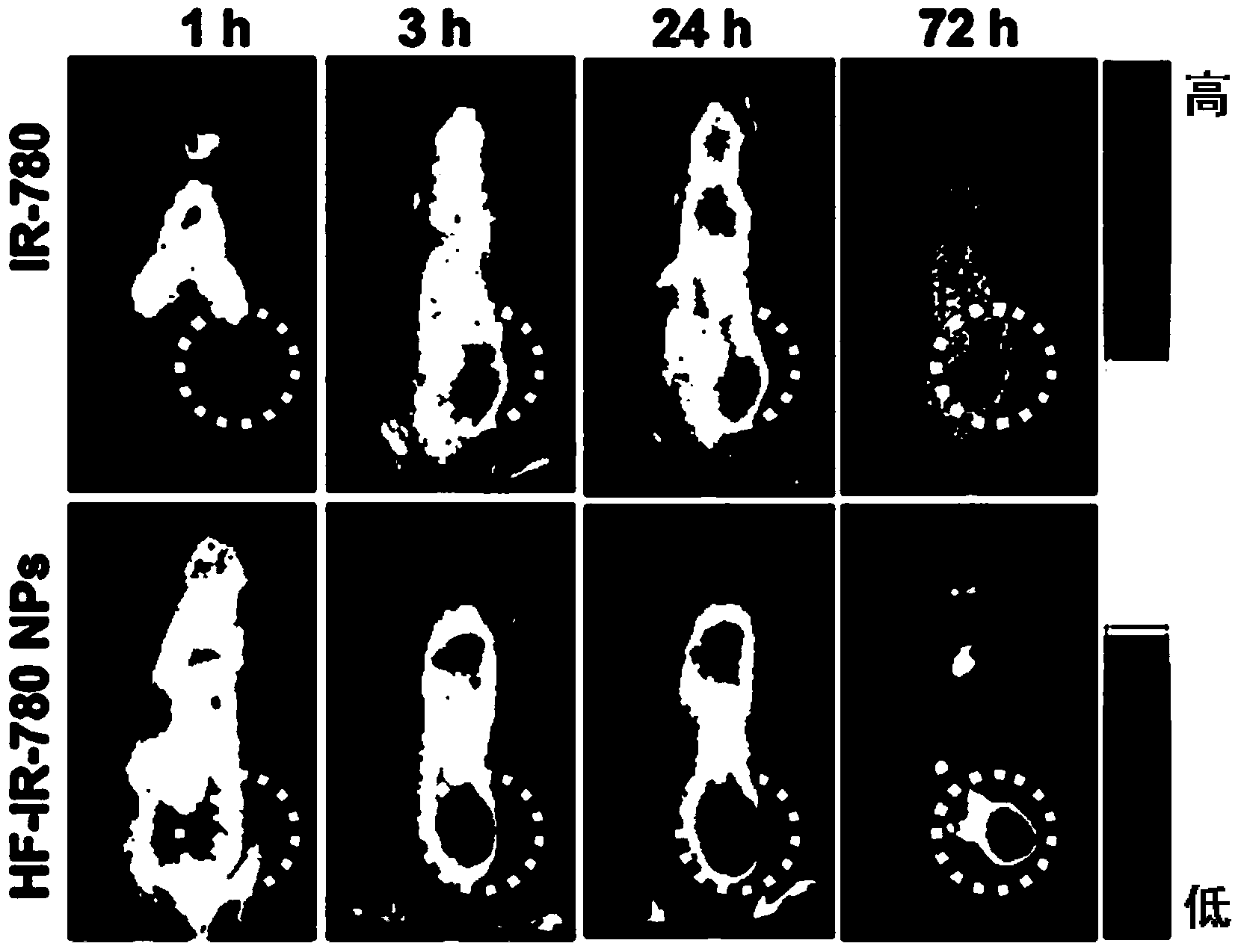
Nano photosensitive drug taking amphiphilic polysaccharide-folic acid conjugate as carrier and preparation method thereof
An amphiphilic polysaccharide and photosensitizing drug technology, applied in the field of nanophotosensitive drugs and their preparation, can solve the problems of poor anti-tumor effect, low bioavailability, and difficulty in dissolving, and achieves good stability and biocompatibility , The effect of good light intensity stability and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] A kind of preparation method of the nano medicine that takes amphipathic polysaccharide-folate conjugate as carrier, comprises the following steps:
[0074] S1. Take the polysaccharide or derivatized polysaccharide, 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) and N-hydroxysuccinimide ( NHS) was dissolved in anhydrous dimethyl sulfoxide, added the derivatized folic acid, and reacted overnight at room temperature under dark conditions, the carboxyl group of the polysaccharide or the carboxyl group formed by derivatization of the polysaccharide and the derivatized carboxyl group formed by the derivatization of folic acid amino group connection to obtain the polysaccharide-folate conjugate solution, after separation and purification, freeze-dry the polysaccharide-folate conjugate solution to obtain a polysaccharide-folate conjugate; the polysaccharide or the derivatized polysaccharide and the The mass ratio of the derivatized folic acid is 1:0.1~1:0....
Embodiment 1
[0125] 1. Dissolve 10g of heparin sodium in 100mL of ultrapure water to form an aqueous solution of heparin sodium, then pass through a cation exchange column, collect the solution after passing through the column, add tri-n-butylamine to adjust the pH to neutral, and freeze-dry to obtain heparin sodium- Tri-n-butylamine complex (expressed as H-tBu);
[0126]2. Dissolve 10g of H-tBu, 25g of succinic anhydride, and 2g of DMAP in anhydrous N'N-dimethylformamide to obtain a mixed solution, react at room temperature for 24 hours, and then put the mixed solution in a dialysis bag with a molecular weight cut off (MWCO) of 3500 After three days of dialysis, take the solution in the dialysis bag and pass it through a cation exchange column, collect the solution after passing through the column, then add tetrabutyl ammonium hydroxide to adjust the pH to neutral, and freeze-dry to obtain derivatized heparin sodium (H-Su) ;
[0127] 3. Take 1g of folic acid and 0.404g of CDI, dissolve i...
Embodiment 2
[0133] 1. Dissolve 10g of dextran, 1g of succinic anhydride, and 1g of DMAP in anhydrous DMSO to obtain a mixed solution, react at room temperature for 12 hours, dialyze the mixed solution in a dialysis bag with a MWCO of 3500 for 3 days, and take the solution in the dialysis bag Freeze drying to obtain derivatized dextran (D-Su);
[0134] 2. Take 1g of folic acid and 0.404g of CDI, dissolve them in 30mL of anhydrous dimethyl sulfoxide and react for 4h, add 0.75mL of ethylenediamine, and react overnight to obtain a derivatized folic acid solution, and then put the derivatized folic acid solution into Added into ethyl acetate, the precipitate was collected by centrifugation, and dried in vacuo to obtain derivatized folic acid (folate-NH 2 ); the volume of ethyl acetate is 10 times the volume of the derivatized folic acid solution;
[0135] 3. Take 2g D-Su, 0.1g EDC.HCL, 0.02g NHS, dissolve in 30mL anhydrous dimethyl sulfoxide for activation, add 0.2g Folate-NH 2 , protected f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com