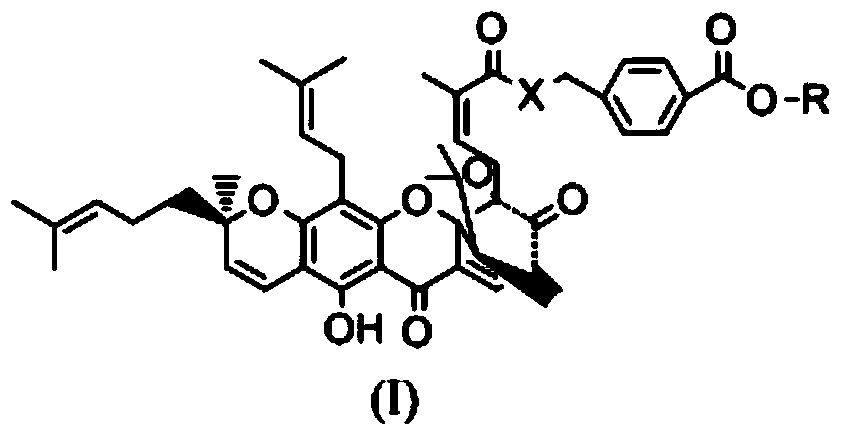
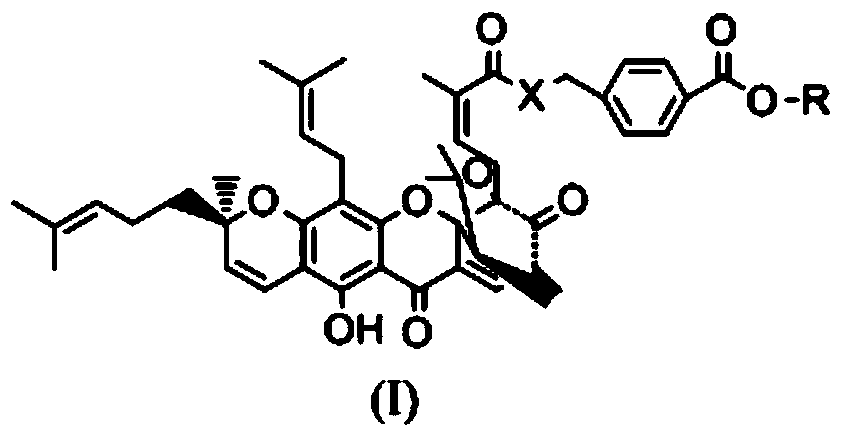
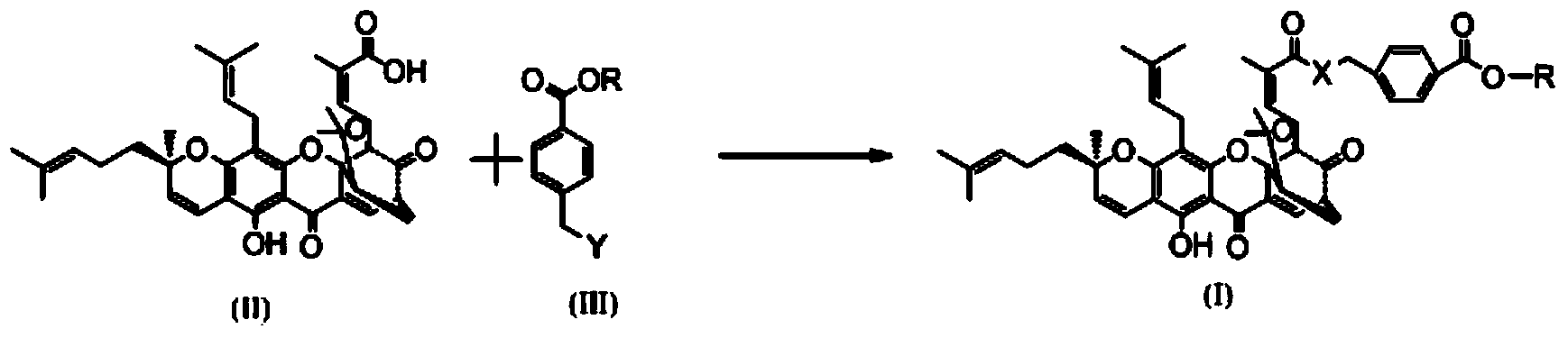Gambogic acid derivative, preparation method and uses thereof
A derivative, the technology of gambogic acid, applied in the field of natural product derivatives of traditional Chinese medicine, can solve the problems of adverse reactions and immunosuppression, and achieve the effect of balancing water solubility and fat solubility, stabilizing chemical structure, and improving fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of WMQ-22
[0037] (1) In a 50ml reaction flask, add 500mg of p-aminobenzoic acid and 20ml of methanol, stir to dissolve, and then slowly add 5ml of SOCl in an ice-water bath. 2 , Install CaCl 2 Dry the tube and react for 3 hours. Then spin dry the solvent, wash repeatedly with distilled water to remove too much unreacted SOCl 2 Wash it off and dry it to obtain methyl para-aminobenzoate hydrochloride.
[0038] (2) In another 50ml reaction flask, add 1.873g of gambogic acid and 10ml of anhydrous dichloromethane and stir together, then add 610mg EDCI, 429mg HOBT, 1g DIEA at room temperature, and finally add 640mg p-aminomethyl benzoate Ester hydrochloride, install a drying tube, stir and react at room temperature for 4 hours. Detection by TCL showed that the reaction was complete. The reaction was quenched with 60 ml of ethyl acetate and washed with saturated brine three times to obtain the crude product of the title compound. The crude product was separated with e...
Embodiment 2
[0041] Synthesis of WMQ-29
[0042] (1) In a 50ml reaction flask, add p-chloromethylbenzoic acid and 10ml ethanol, stir to dissolve, and then slowly add 2ml SOCl dropwise in an ice-water bath 2 , Install CaCl 2 Dry the tube and react for 3 hours. Then spin dry the solvent, wash repeatedly with distilled water to remove too much unreacted SOCl 2 Wash it off and dry it to obtain ethyl p-chloromethyl benzoate.
[0043] (2) In another 50ml reaction flask, add 500mg of gambogic acid and 10ml of anhydrous dichloromethane and stir together, then add sodium bicarbonate powder and methyl p-chloromethyl benzoate at room temperature, install a drying tube, Stir overnight at room temperature. Detection with TCL showed that the reaction was complete. The reaction was quenched with 50ml of dichloromethane and washed twice with 10ml of saturated sodium bicarbonate solution. Then, it was washed three times with saturated brine to obtain a crude product of the title compound. The crude product w...
Embodiment 3
[0050] In vitro anti-tumor activity test
[0051] 1. Experimental cell line:
[0052] The tumor cell lines used in this experiment are: A549 (human lung adenocarcinoma cells), HCT116 (human colon cancer cells), ZR-75-30 (human breast cancer cells) and HepG2 (human liver cancer cells) (by Shanghai Pharmaceutical Industry Cryopreservation and passage in the Pharmacology Laboratory of the Institute).
[0053] 2. Sample preparation:
[0054] After dissolving with DMSO (Merck), add PBS(-) to make a 1000μg / ml solution or uniform suspension, and then dilute with PBS(-) containing DMSO. The positive control drug is gambogic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com