Preparation method of fipronil
A technology of fipronil and trifluoromethyl phenyl, which is applied in the field of chemical synthesis, can solve the problems of reduced fipronil content and yield, large amount of trifluoroacetic acid, and a large amount of dilute sulfuric acid waste water, etc., to ensure purity, reduce The effect of reducing the amount of production process cost and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
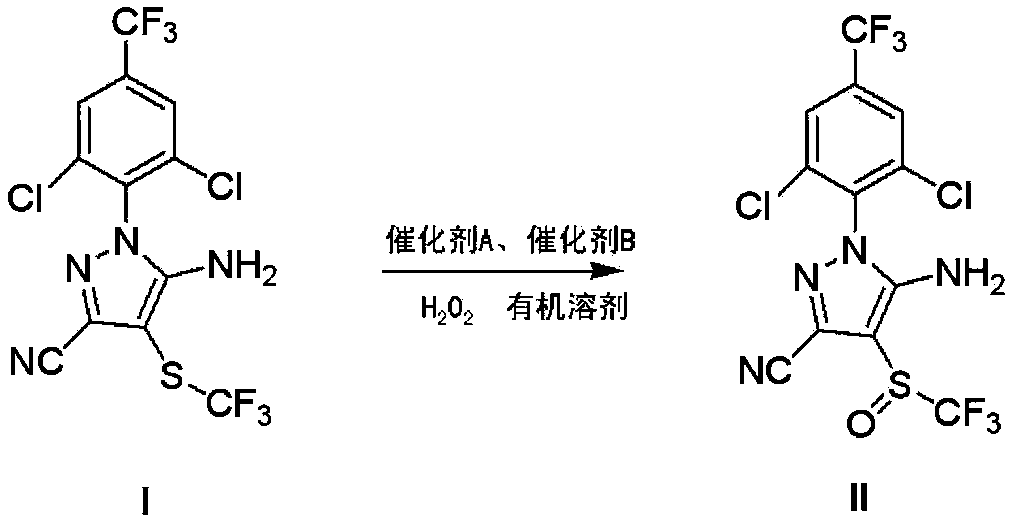
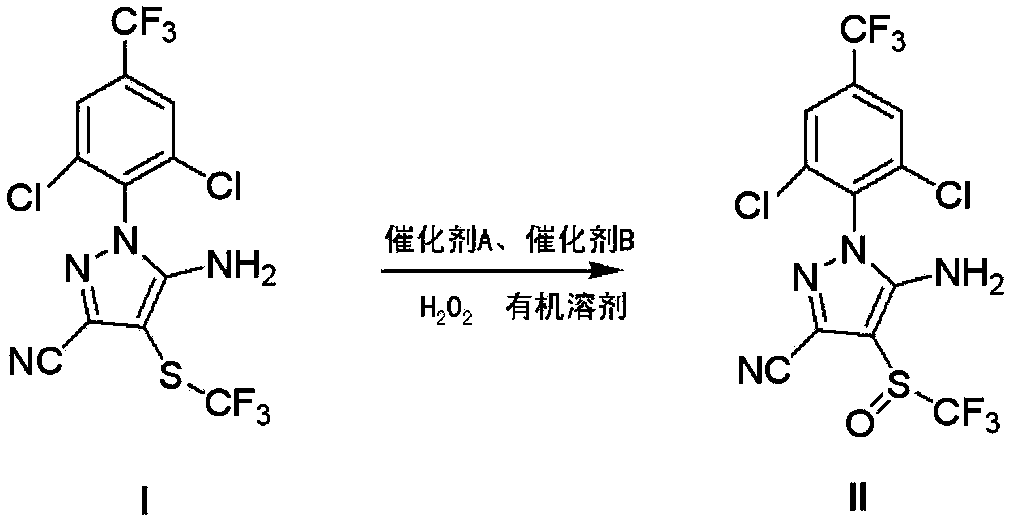
[0029] The preparation of embodiment 1 fipronil
[0030] 105.2 kg (237 mol) of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyrazole (95% pure, Zhejiang Hisun Chemical Co., Ltd.) mixed with 450kg (4545.45mol) dichloroethane, then added 6kg (52mol) trifluoroacetic acid and 12kg (122mol) concentrated sulfuric acid at one time, and the reaction temperature was controlled at 5-35°C , directly add 20.2kg of 50% (297mol) hydrogen peroxide. Continue stirring, when the reaction conversion rate is 67% (HPLC detection, control the mass fraction of peroxidized impurities to be less than 1%), add an aqueous solution with a mass fraction of 10% sodium sulfite to quench the reaction until the starch potassium iodide test paper does not turn blue, and then use mass fraction The pH value was adjusted to 6-7 with a 12% sodium hydroxide solution, and the solid obtained after suction filtration was crystallized 4 times with dichloroethane to obtain 61.2 kg of pu...
Embodiment 2
[0032] The preparation of embodiment 2 fipronil
[0033] 105.2 kg (237 mol) of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyrazole (95% pure, Zhejiang Hisun Chemical Co., Ltd.) and 450kg (4545.45mol) of dichloroethane were mixed and stirred, and then 1.2kg (10mol) of trifluoroacetic acid and 4.8kg (48mol) of concentrated sulfuric acid were added at one time. Control the temperature at 5-35°C, and then add 20.2kg of 50% (297mol) hydrogen peroxide. Continue to stir until the reaction conversion rate reaches 62% (HPLC detection, control the mass fraction of peroxidized impurities to be less than 1%), add an aqueous solution with a mass fraction of 10% sodium sulfite to quench the reaction until the starch potassium iodide test paper does not turn blue, and then use a mass fraction of 12% sodium hydroxide solution was used to adjust the pH value to 6-7. After suction filtration, the solid was crystallized with dichloroethane for 6 times to obtai...
Embodiment 3
[0035] The preparation of embodiment 3 fipronil
[0036]105.2 kg (237 mol) of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyrazole (95% pure, Zhejiang Hisun Chemical Co., Ltd.) and 600kg (5330mol) of chlorobenzene were mixed and stirred, and then 6kg (52mol) of trifluoroacetic acid and 12kg (122mol) of concentrated sulfuric acid were added at one time. Control the temperature at 5-35°C, and then add 20.2kg of 50% (297mol) hydrogen peroxide. Continue to stir until the reaction conversion rate reaches 70% (HPLC detection, control the mass fraction of peroxidized impurities to be less than 1%), add an aqueous solution with a mass fraction of 10% sodium sulfite to quench the reaction until the starch potassium iodide test paper does not turn blue, and then use a mass fraction of 12% sodium hydroxide solution was used to adjust the pH value to 6-7. After suction filtration, the solid was crystallized with dichloroethane for 5 times to obtain 59.1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com