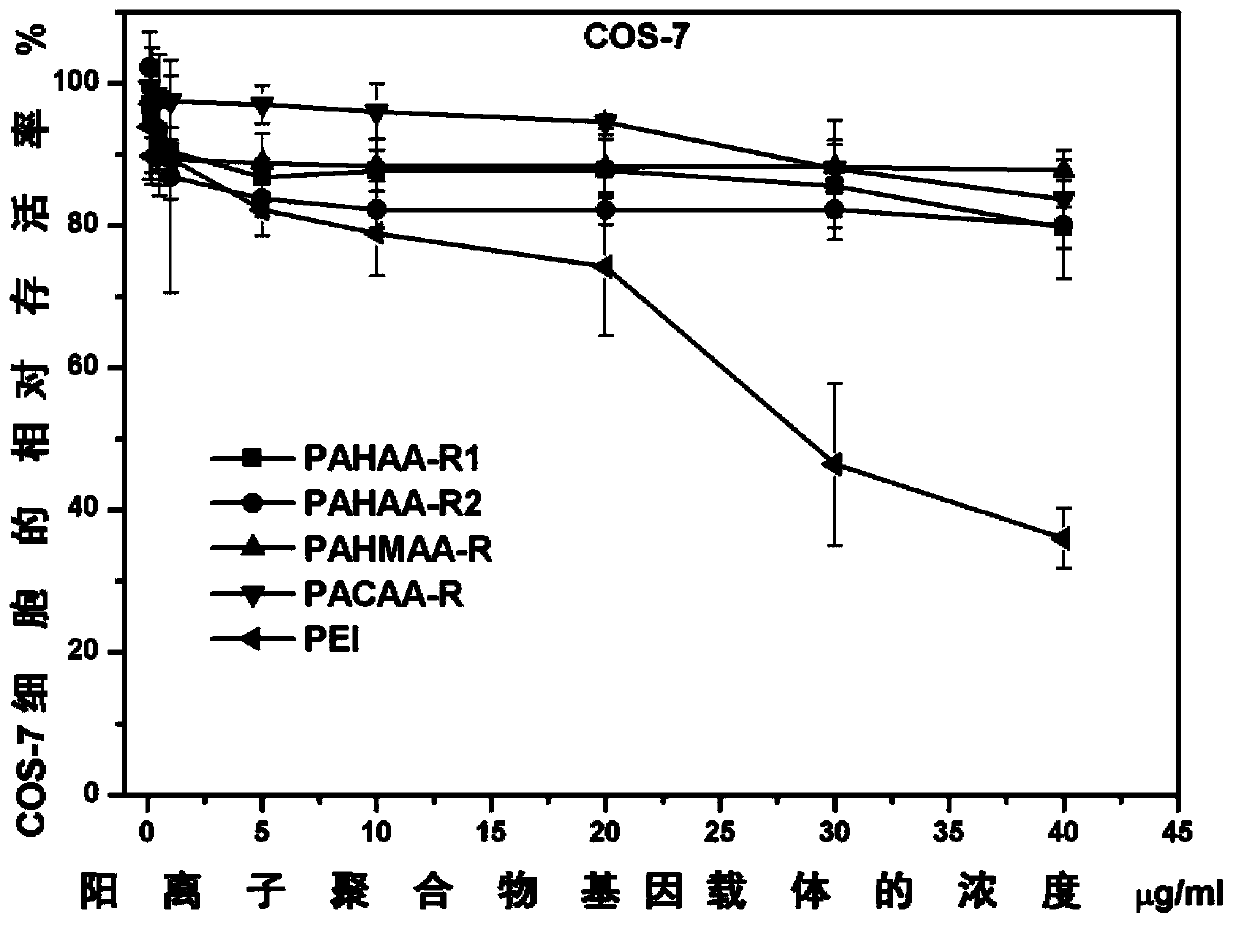
Poly(methyl)acrylamide cationic polymer modified by natural arginine on side chain, preparation method and application
A technology of acrylamide cationic polymers, applied in the field of poly(meth)acrylamide cationic polymers and gene delivery carriers, can solve the problem of low transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The first step: Add 0.26g (2.2mmol) 1,6‐hexanediamine and 0.48g (2.2mmol) di-tert-butyl dicarbonate, 0.5ml triethylamine, 25ml methanol into the reaction flask, at 20°C The reaction was stirred for 20 min. The solvent methanol was removed by rotary evaporation under reduced pressure, and 10ml of 1M NaH 2 PO 4 The solution dissolves the residue, and washes twice with 15 ml of ether. Then adjust the pH value of the aqueous phase to above 9 with 1M NaOH solution, extract 4 times with 15 ml of ethyl acetate, combine the organic phases, and dry with anhydrous sodium sulfate. Finally, the solvent was removed by rotary evaporation under reduced pressure and purified to obtain hexamethylenediamine protected by a single-terminal amino Boc in a transparent oil with a yield of 35%. Its main chemical structural features are as follows:
[0042] 1 H NMR (CDCl 3 ):3.11(‐CH 2 NHBoc), 2.67(‐CH 2 NH 2 ), 1.55‐1.27 (4×CH 2 ,C(CH 3 ) 3 )
[0043] The second step: Fully dissolv...
Embodiment 2
[0054] tert-butoxycarbonyl‐N ω ‐(2,2,4,6,7‐pentamethyldihydrobenzofuran‐5‐sulfonyl)‐L‐arginine (Boc‐Arg(Pbf)‐OH 354 mg), benzotriazole‐N ,N,N',N'-Tetramethyluronium hexafluorophosphate (HBTU254mg), diisopropylethylamine (DIPEA160μL) were dissolved in 15ml DMF solution, stirred and reacted at 20°C for 30min, and added to Example 1 The four-step reaction obtained 250 mg of acrylamide polymer product with a degree of polymerization of 28 and containing side group terminal amino functional groups, and continued to stir and react at 20° C. for 3 days. The organic solvent was removed by rotary evaporation under reduced pressure, a mixture of 5 ml of dichloromethane and 5 ml of trifluoroacetic acid was added, and the reaction was stirred at 20° C. for 1 h. Then remove the organic solvent under reduced pressure, then add 25ml of water to mix and filter, dialyze the resulting filtrate in pure water for 2 days, and further freeze-dry to obtain the polyacrylamide cationic functional pol...
Embodiment 3
[0059] The acryloyl chloride in Example 1 was changed to methacryloyl chloride, and the same reaction conditions and preparation steps as in Example 1 were maintained to synthesize the obtained hexamethylenediamine single-terminal amino Boc-protected methacrylamide polymer. pass 1 H nuclear magnetic resonance compares the characteristic peak integral of RAFT reagent and the characteristic peak integral of monomer, calculates that its degree of polymerization is 33, and its number average molecular weight is M n,NMR =9.7KDa. The number molecular weight was measured by gel permeation chromatography (GPC) with tetrahydrofuran as the mobile phase. n,GPC =10.7KDa, PDI is 1.16. Further, the obtained polymer product was dissolved in a mixture of 5 ml of dichloromethane and 0.5 ml of hydrochloric acid, stirred for 1 h, concentrated by rotary evaporation under reduced pressure, precipitated in glacial ether, filtered, and dried in a vacuum oven to prepare A methacrylamide polymer co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com