Method for catalytically synthesizing tamibarotene through acenaphthene imidazole n-heterocyclic carbine allyl palladium chloride compound
A technology of allyl palladium chloride and acenaphthoimidazole nitrogen, which is applied in the field of synthesis of tamibarotene, can solve problems such as non-compliance with environmental protection, increase the economic cost of the synthesis route, and large environmental pollution, and achieve great competitive advantages and Industrial production utilization value, avoiding irritating acid chloride and acid waste, and the effect of cheap and easy-to-obtain synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
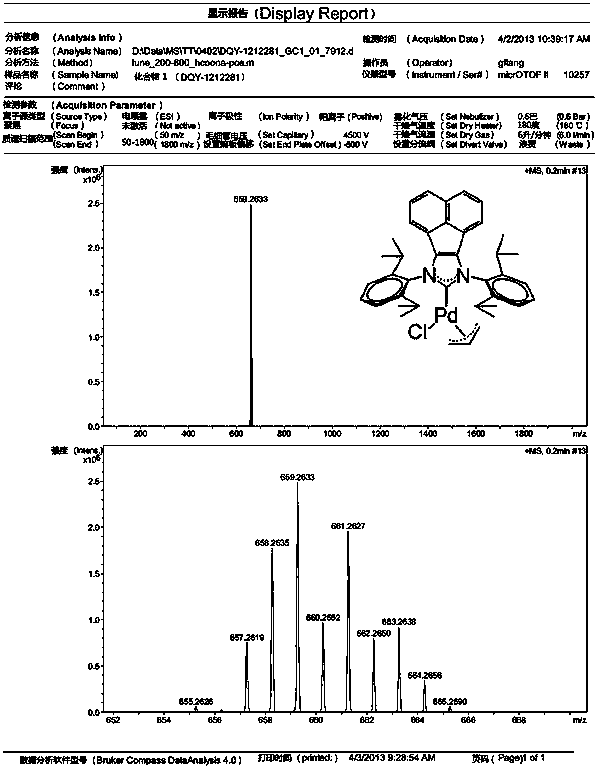
[0048] Example 1: Novel acenaphthoimidazole nitrogen heterocyclic carbene allyl palladium chloride catalyst 1 Preparation of:
[0049] Under nitrogen, acenaphthoimidazole hydrochloride (1.26 g, 2.3 mmol), allyl palladium chloride dimer (0.4 g, 1.1 mmol), potassium tert-butoxide were added sequentially to a 50 mL round bottom flask (0.31 g, 2.7 mmol) and tetrahydrofuran (24 mL). Stir the reaction at room temperature for 24 hours, remove the solvent under reduced pressure, separate by column chromatography, and dry in vacuum to obtain a yellow solid, which is the acenaphthoimidazole nitrogen heterocyclic carbene allyl palladium chloride catalyst 1 . Yield: 1.35 g, 88%.
[0050] NMR analysis: 1 H NMR (CDCl 3 , 400 MHz, 298 K): δ = 7.69 (d, J = 8.4 Hz, 2H), 7.54 (t, J = 8.0 Hz, 2H), 7.39 – 7.32 (m, 6H), 6.84 (d, J = 6.8 Hz, 2H), 4.97 – 4.87 (m, 1H), 3.98 (d, J = 7.2 Hz, 1H), 3.37 – 3.27 (m, 3H), 3.16 – 3.10 (m, 2H), 2.90 (d, J = 13.2 Hz, 2H), 1.86 (d, J = 11.6 ...
Embodiment 2
[0052] Example 2: Preparation of ethyl 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)carbamoyl]benzoate (1):
[0053] Under nitrogen, add potassium phosphate (0.741 g, 3.5 mmol), acenaphthoimidazole nitrogen heterocyclic carbene allyl palladium chloride catalyst to a 50 mL round bottom flask in sequence 1 (0.006 g, 1 mol%), 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine (0.53 g, 2.61 mmol), ethyl p-iodobenzoate (0.241 g, 0.87 mmol) and toluene (6 mL). Pass carbon monoxide gas into the reaction mixture for 30 seconds, put the reaction bottle into an oil bath, and heat to 90°C for 19 hours. During the reaction, a carbon monoxide balloon was used to maintain the gas pressure in the reaction flask at 1 atmosphere. After the reaction, the solvent was removed under reduced pressure, separated by column chromatography, and dried in vacuum to obtain a white solid, namely 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl -2-Naphthyl)carbamoyl]benzoic acid ethyl ester. Yi...
Embodiment 3
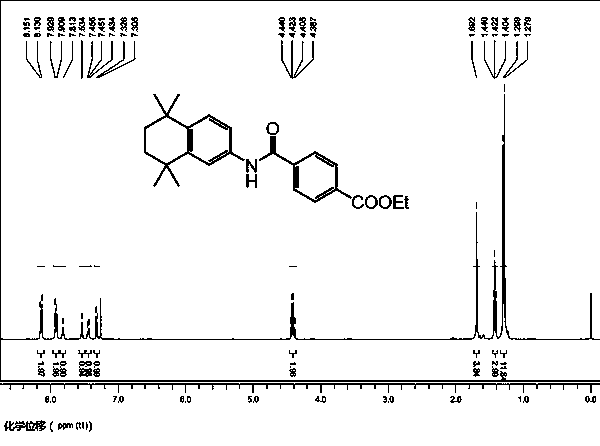
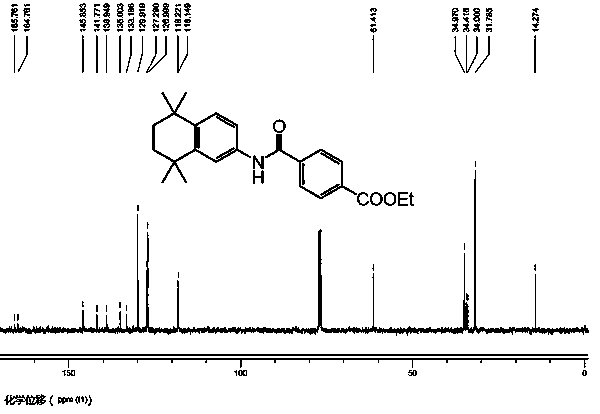
[0058] Example 3: Preparation of ethyl 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)carbamoyl]benzoate (2):
[0059] Under nitrogen, add potassium phosphate (8.5 g, 40 mmol), acenaphthoimidazole nitrogen heterocyclic carbene allyl palladium chloride catalyst to a 200 mL round bottom flask in sequence 1 (0.068 g, 1 mol%), 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine (6.08 g, 29.9 mmol), ethyl p-iodobenzoate (2.76 g, 10 mmol) and toluene (70 mL). Pass carbon monoxide gas into the reaction mixture for 1 minute, put the reaction bottle into an oil bath, and heat to 90°C for 24 hours. During the reaction, a carbon monoxide balloon was used to maintain the gas pressure in the reaction flask at 1 atmosphere. After the reaction was completed, column chromatography separated and vacuum dried to obtain a white solid, namely 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl ) ethyl carbamoyl]benzoate. Yield: 3.34 g, 92%.
[0060] NMR analysis: 1 H NMR (CDCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com