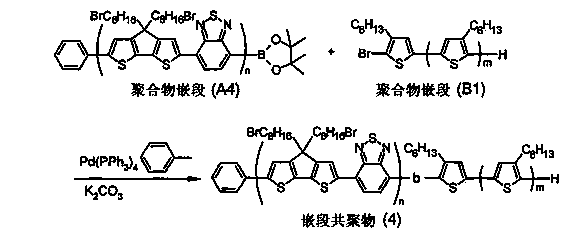
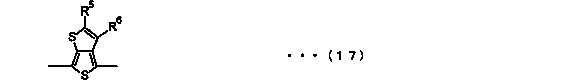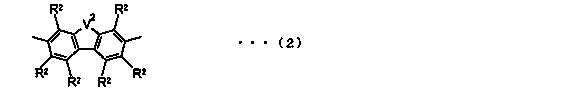Block copolymer and photoelectric conversion element
A technology of block copolymers and polymers, which can be used in electrical components, electrical solid devices, circuits, etc., and can solve problems such as weak light absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0174] Examples of the present invention are described in detail, but the scope of the present invention is not limited by these Examples.
[0175] The measurement and purification methods of the physical properties of the materials produced in the above respective steps and in the following steps were performed as follows.
[0176] [Weight average molecular weight (Mw), number average molecular weight (Mn)]
[0177] Based on condensate obtained in polystyrene equivalent values from a GPC apparatus (HLC-8020, trade name, manufactured by Tosoh Corporation) having two columns connected in series (trade name, TSKgel Multipore HZ, manufactured by Tosoh Corporation) Gel Permeation Chromatography (GPC) measurements determine the number average molecular weight and weight average molecular weight. The measurement was performed at 40° C. using chloroform as a solvent.
[0178] [Purification of polymers]
[0179] Purification of the obtained polymer was carried out using a prepara...
Synthetic example 1
[0185] A monomer represented by the following formula (i) was synthesized.
[0186]
[0187] Under a nitrogen atmosphere, cyclopentadieno[2,1-b:3,4-b']dithiophene (0.36 g, 2.0 mmol) and tetrahydrofuran (30 mL) were added to a 100 mL three-necked flask and cooled to below 0°C. Then a 1.6M solution of n-butyllithium in hexane (1.38 mL, 2.2 mmol) was slowly added dropwise, and stirred for 1 hour after the temperature rose to room temperature. After cooling the temperature to 0°C again, 8-bromo-1-iodo-octane (0.64 g, 2.0 mmol) was added, followed by stirring for 1 hour. A 1.6 M solution of n-butyllithium in hexane (1.38 ml, 2.2 mmol) was then slowly added dropwise. After raising the temperature to room temperature, stirring was continued for 1 hour. Cool the temperature below 0°C. Then 8-bromo-1-iodo-octane (0.64 g, 2.0 mmol) was added, followed by stirring for 1 hour. After completing the reaction, the reaction mixture was poured into saturated brine (100 mL) and extracte...
Synthetic example 2
[0190] A monomer represented by the following formula (ii) was synthesized.
[0191]
[0192] Under a nitrogen atmosphere, the compound represented by formula (i) (0.62 g, 1.1 mmol) and tetrahydrofuran (13 mL) were charged into a 100 mL three-necked flask, and then cooled to below 0°C. Subsequently, N-bromosuccinimide (0.39 g, 2.2 mmol) was added slowly, followed by stirring at below 0°C for 1 hour. Raise the temperature to room temperature. After completing the reaction, the reaction mixture was poured into saturated brine (100 mL), followed by extraction with hexane (30 mL×3), followed by washing with water (30 mL×3). The obtained organic layer was dried over sodium sulfate, and then the solvent was evaporated under reduced pressure to obtain a crude product, which was purified by silica gel column chromatography (hexane) to obtain 2, as a yellow solid represented by formula (ii). 6-Dibromo-4,4-bis(8-bromooctyl)cyclopentadieno[2,1-b:3,4-b']dithiophene (0.7 g, 88%).
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com