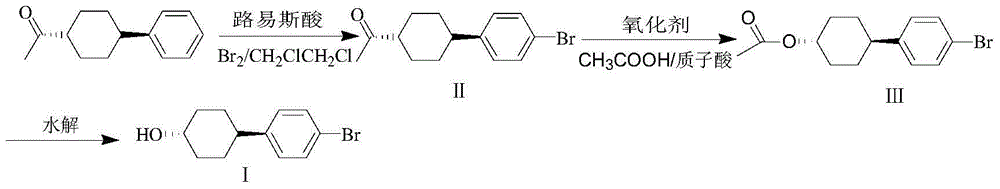
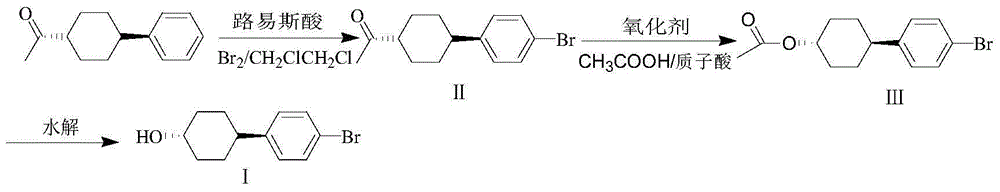
Preparation method of trans-4,4'-(1-bromophenyl)-cyclohexanol
A technology of cyclohexanol and bromophenyl, applied in the field of organic compound preparation, can solve problems such as unfavorable industrial production, low product yield, potential safety hazards, etc., achieve large-scale industrial production, high quality yield, and overcome high cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Under the protection of nitrogen (30ml / min) at 20-30°C, trans-1-(4-phenyl-cyclohexyl)-ethanone (0.8mol, 161.6g), aluminum trichloride (0.8 mol, 106.8g), ferric chloride (0.24mol, 38.9g), dichloroethane (1100g). After stirring and cooling down to 2°C, bromine (1.2mol, 192g) was added dropwise, and the temperature was controlled at -5-10°C. During the dropwise addition, nitrogen gas (30ml / min) was used to discharge the hydrogen bromide produced by the reaction into the tail gas absorption system. After 1-3 hours of dripping, keep warm at -5-10°C for 2 hours. After the heat preservation is over, pour the reaction solution into ice water dissolved in NaOH (0.5mol, 20g) for hydrolysis. After liquid separation, the obtained organic phase was washed with water until pH = 7, and the solvent was removed to obtain 230.3 g of crude product, GC: 85.0%, and 175.5 g of II, GC: 99.1%. Yield 78%. mp: 58~60℃. MS (m / Z): 282 (M + ), 280 (M + ), 264, 262, 237, 235, 184, 182, 171, 169...
Embodiment 2
[0033]Under the protection of nitrogen (30ml / min) at 20-30°C, put 1-(4-phenyl-cyclohexyl)-ethanone (0.8mol, 161.6g), aluminum trichloride (0.8mol, 106.8 g), ferric chloride (0.16mol, 26g), dichloroethane (1100g). After stirring and cooling down to 2°C, bromine (1.2mol, 192g) was added dropwise, and the temperature was controlled at -5-10°C. During the dropwise addition, nitrogen gas (30ml / min) was used to discharge the hydrogen bromide produced by the reaction into the tail gas absorption system. After 1-3 hours of dripping, keep warm at -5-10°C for 2 hours, and then pour the reaction solution into ice water dissolved in NaOH (0.5mol, 20g) for hydrolysis. After liquid separation, the organic phase was washed with water until pH = 7, and the solvent was removed to obtain 231.1 g of crude product, GC: 82.5%, and 171.0 g of II, GC: 99.0%. Yield 76%. mp: 58~60℃. MS (m / Z): 282 (M + ), 280 (M + ), 264, 262, 237, 235, 184, 182, 171, 169, 154, 143, 129, 116, 103, 90, 71, 55, 51....
Embodiment 3
[0036] Under the protection of nitrogen (30ml / min) at 20-30°C, put 1-(4-phenyl-cyclohexyl)-ethanone (0.8mol, 161.6g), aluminum trichloride (0.8mol, 106.8 g), ferric chloride (0.4mol, 64.9g), dichloroethane (1100g). After stirring and cooling down to 2°C, bromine (1.2mol, 192g) was added dropwise, and the temperature was controlled at -5-10°C. During the dropwise addition, nitrogen gas (30ml / min) was used to discharge the hydrogen bromide produced by the reaction into the tail gas absorption system. After 1-3 hours of dripping, keep warm at -5-10°C for 2 hours, and then pour the reaction solution into ice water dissolved in NaOH (0.5mol, 20g) for hydrolysis. After liquid separation, the obtained organic phase was washed with water until pH = 7, and the solvent was removed to obtain 225.1 g of crude product, GC: 81.7%, and 164.2 g of II, GC: 98.9%. Yield 73%. mp: 58~60℃. MS (m / Z): 282 (M + ), 280 (M + ), 264, 262, 237, 235, 184, 182, 171, 169, 154, 143, 129, 116, 103, 90, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com