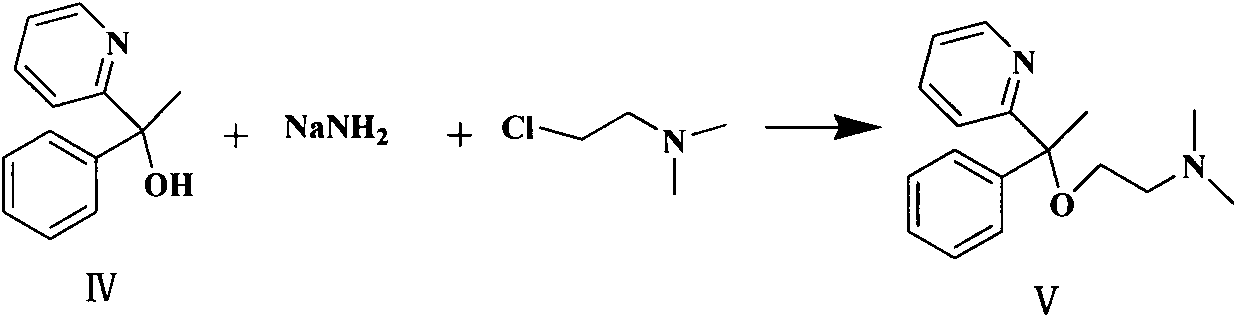
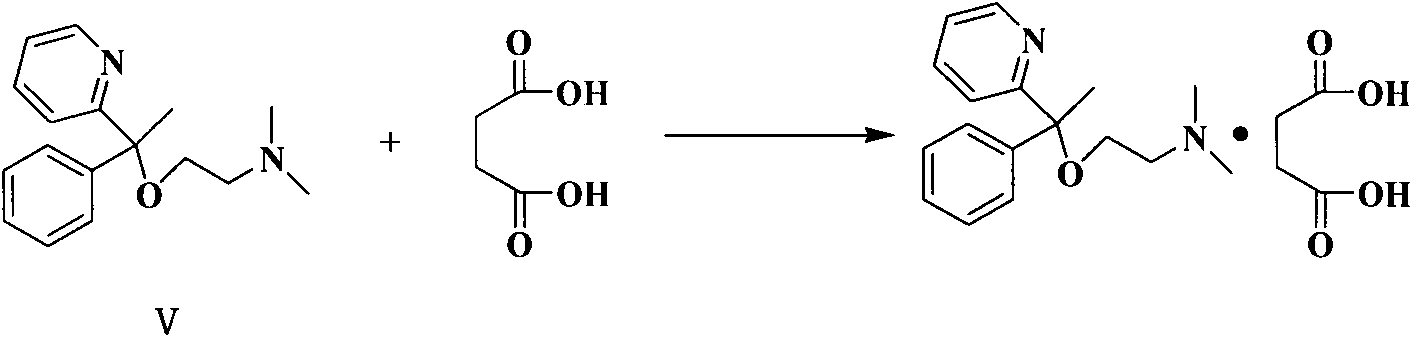Preparation method of doxylamine succinate
A technology of succinic acid and Westminster, which is applied in the field of medical technology and organic synthesis, can solve the problems of complex post-processing, low reaction efficiency, and difficulty in ensuring product yield and purity, and achieve simple post-processing, high reaction efficiency, and product yield. The effect of high rate and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
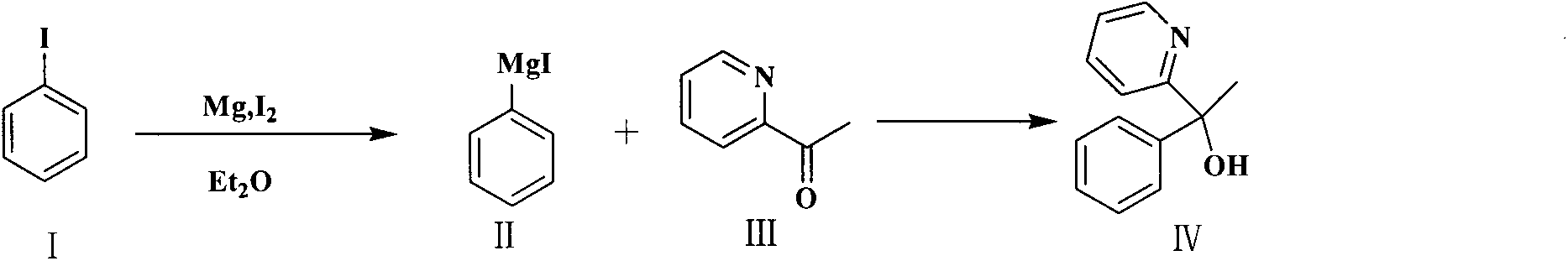
[0032] Example 1 Preparation of 2-pyridylphenylmethylcarbinol
[0033]
[0034] Add 13.92g (0.58mol) of magnesium chips activated by dilute hydrochloric acid into a 1000ml three-neck flask, add 300ml of ether and 1 grain of iodine; then slowly drop 114.24g (0.56mo) of iodobenzene into 100ml of ether and drop it into 10ml, wait until the red The brown color turned into light yellow and began to reflux, and the remaining iodobenzene was added dropwise, and the solution changed from light yellow to milky white, and gradually turned gray again. After the drop, stirred and refluxed for 15 hours, 48.20 g (0.4 mol) of 2-acetylpyridine Mix 100ml of diethyl ether and slowly drop into the above solution, the solution turns from gray to yellow, continue to react for 2-3 hours after the drop, after the reaction is completed, add dropwise 100ml of saturated NH 4 Cl aqueous solution, stir while adding, continue to stir for 1h after dripping, separate and retain the organic phase, extract...
Embodiment 2
[0035] Example 2 Preparation of 2-pyridylphenylmethylcarbinol
[0036] Add 13.92g (0.58mol) of magnesium chips activated by dilute hydrochloric acid into a 1000ml three-necked flask, add 300ml of methyl tert-butyl ether and 1 grain of iodine; then mix 114.24g (0.56mo) of iodobenzene with 100ml of methyl tert-butyl ether Slowly add 10ml of butyl ether, wait until the reddish-brown color turns light yellow and starts to reflux, add the remaining iodobenzene dropwise, the solution changes from light yellow to milky white, and gradually turns gray again, stir and reflux for 10 hours after dropping, and then 48.20g (0.4mol) of 2-acetylpyridine mixed with 100ml of methyl tert-butyl ether was slowly dropped into the above solution, the solution turned from gray to yellow, and continued to react for 2 to 3 hours after the completion of the reaction. After the reaction was completed, 100ml of saturated NH 4 Cl aqueous solution, stir while adding, continue to stir for 1h after dripping...
Embodiment 3
[0037] Example 3 Preparation of 2-pyridylphenylmethylmethanol
[0038] Add 7.2g (0.30mol) of magnesium chips activated by dilute hydrochloric acid into a 1000ml three-necked flask, add 100ml of tetrahydrofuran and 1 grain of iodine; then slowly drop 50.0g (0.25mo) of iodobenzene mixed with 50ml of tetrahydrofuran into 10ml, wait until the red The brown color turns light yellow and starts to reflux, the remaining iodobenzene is added dropwise, the solution turns from light yellow to milky white, and gradually turns gray again, after dropping, stir and reflux for 8 hours; Mix 50ml of tetrahydrofuran and slowly drop it into the above solution, the solution turns from gray to yellow, and continue to react for 2-3 hours after the drop; after the reaction is completed, add 100ml of saturated NH 4 Cl aqueous solution, stir while adding, continue to stir for 1h after dropping, separate and retain the organic phase, extract the water phase with 2×200ml tetrahydrofuran once, combine the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com