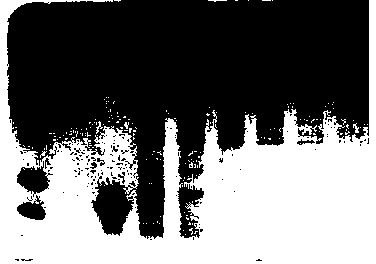Recombinant ganoderma lucidum immunoregulatory protein, human serum albumin fusion protein, and preparation method and application thereof
An immunomodulatory protein, human serum albumin technology, applied in the direction of serum albumin, albumin peptides, fusion of prolonging plasma life, etc., can solve the problems of high clearance rate, difficult to meet pharmacokinetic parameters, etc. rate, high yield, and the effect of reducing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Construction and expression of rLZ-8 fusion protein engineering strain
[0025] Construction parts: According to the codon preference of yeast, the sequences of the target fragments rLZ8-HSA, rLZ8-Fc1 and rLZ8-Fc4 were respectively synthesized and stored in the puc57 plasmid. Design primers containing restriction sites StuI and KpnI. Primers were synthesized as follows:
[0026] (1) LZ-8-HSA: 5' CATAGGCCTTCTGATACTGCTTTGA 3'
[0027] 5' CGGGGTACCGAATTCCTATTACA 3'
[0028] (2) LZ-8-Fc1: 5' GTTAGGCCTTCTGATACTGCTTTGA 3'
[0029] 5' TAGGGTACCTCATTTACCAGGGG 3'
[0030] (3) LZ-8-Fc4: 5' CCGAGGCCTTCTGATACTGCTT 3'
[0031] 5' GATGGTACCTCACGGAGCATGAG 3'
[0032] The target fragment was obtained by PCR. The PCR conditions were: start at 95°C for 30s, then 95°C for 30s, 58°C for 30s, 72°C for 2min, a total of 30 cycles, and finally 72°C for 10min and 16°C, standby.
[0033] 1% agarose electrophoresis identified target fragments at 2184bp (LZ-8-HSA), 1064bp (LZ-8-Fc...
Embodiment 2
[0037] Example 2 Fusion protein purification process
[0038] Since the fusion protein has a common LZ-8 structure, gravity column affinity chromatography, molecular sieve, immobilized metal chelate affinity chromatography (IMAC) method, hydrophobic chromatography ( HIC), anion exchange chromatography and other methods of purification, the specific methods are as follows:
[0039] Purification method of rLZ-8-Fc1 and rLZ-8-Fc4:
[0040] Microfiltration: Centrifuge the fermentation broth at 10,000 rpm to obtain the supernatant, then purify it with a hollow fiber column with a pore size of 100Kd (microfiltration), remove small molecule salts and sugars, and obtain 8L of pigment, nucleic acid, and protein Yellow clear liquid.
[0041] rProtein A Gravi Trap gravity column affinity chromatography: preparation buffer phase A: 0.22M phosphate buffer, pH 7.7, 0.15M sodium chloride, preparation buffer phase B: 0.1M citrate buffer, pH 4.0, Suction filtration at 0.22 μm, ultrasonic de...
Embodiment 3
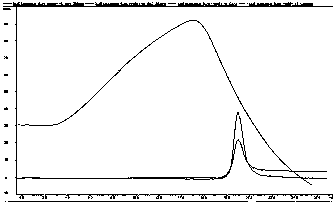
[0050] Example 3 Comparison of different fusion proteins promoting proliferation of mouse splenocytes
[0051] The WST-1 method is used to detect the influence of the fusion protein on the proliferation of mouse splenocytes, which reflects the strength of its biological activity. The present invention adopts BALB / c female mice with a body weight of 20-22g, and the mice are killed by pulling the neck. Take the spleen under aseptic conditions and place it in a plate with 5ml of DMEM containing 10% calf serum in advance; mash the spleen with tweezers, filter the tissue suspension with gauze to remove the tissue pieces, and prepare the spleen cell suspension; Take 100 microliters of cell suspension and add 900 microliters of 2% glacial acetic acid solution to count under the microscope. Adjust the cell concentration to 5×10 with DMEM containing 2% calf serum 6 / ml; respectively prepare the fusion protein and rLZ-8 with the same molar concentration gradient, set 3 gradient concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com