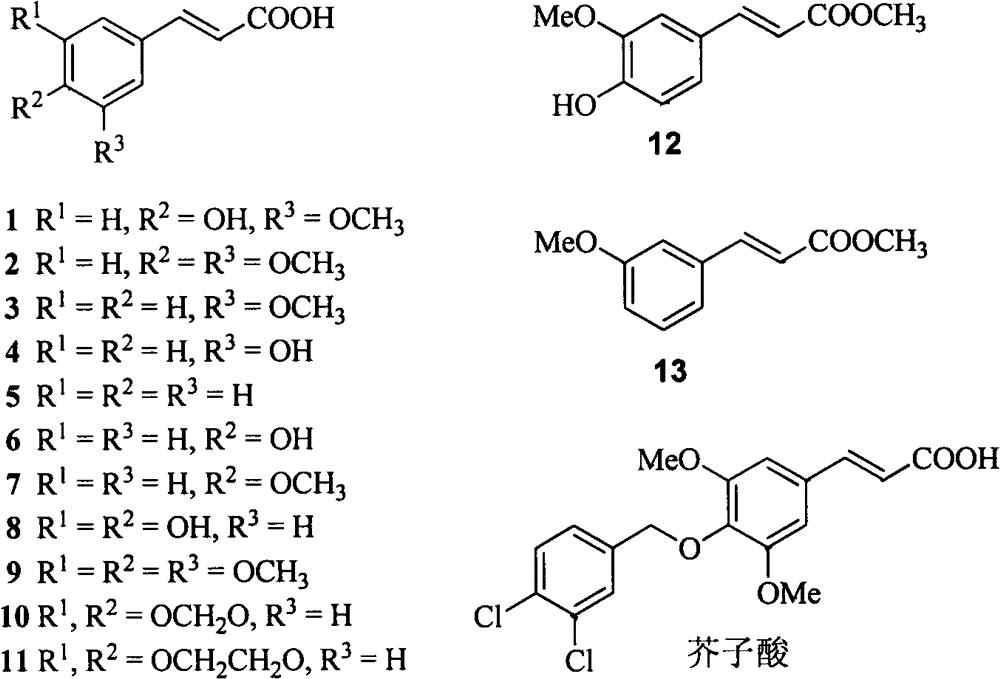
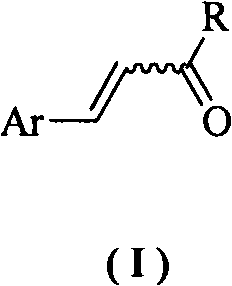
3-Arylacrylic acid and its derivatives anti-plant virus agent
An anti-plant virus agent, anti-plant virus technology, applied in the field of anti-plant virus agent, plant virus disease prevention and control, the field of new anti-plant virus agent, can solve the problems of poor control effect, environmental risk, difficult quality control and the like, Achieve the effect of extremely low toxicity, good environmental compatibility and simple chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Synthesis of 3-Aryl Acrylic Acids 14-22 and 24-29:
[0023]
[0024] A mixture of aromatic aldehyde (2.16mmol), malonic acid (0.45g, 4.32mmol), piperidine (2.0mL) and pyridine (20mL) was heated under reflux for 3-40h. Cool to room temperature, add 25% K to the reaction solution 2 CO 3 solution, heating with stirring, liquid separation, acidification of the aqueous phase with dilute hydrochloric acid, filtration, and drying to obtain the crude product, and recrystallization or column chromatography to obtain the pure product.
[0025] (E)-3-(2-furyl)acrylic acid (14)
[0026] Purified by column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain a yellow solid with a yield of 66%. Melting point 143-144°C;1 HNMR (400MHz, CDCl 3 ): δ7.53(d, 3 J HH =15.6Hz, 1H), 7.52(d, J=1.6Hz, 1H), 6.68(d, J=3.6Hz, 1H), 6.50(dd, J=3.6Hz, 1.6Hz, 1H), 6.32(d, J=15.6Hz, 1H).
[0027] (E)-3-(2-thienyl)acrylic acid (15)
[0028] Purified by column chromatogra...
Embodiment 2
[0056] Embodiment 2: Synthesis of (E)-3-(2-hydroxyl-1-naphthyl)acrylic acid (23)
[0057]
[0058] Formethoxymethyl triphenylphosphine bromide (3.73 g, 8.72 mmol) and 2-hydroxy-1-naphthaldehyde (1.50 g, 8.72 mmol) in methanol (20 mL) were slowly added potassium carbonate (2 g) in methanol (20 mL) solution, the reaction was stirred at room temperature for 6 h, then concentrated. Dichloromethane (50 mL) and water (50 mL) were added, the layers were separated, the aqueous phase was extracted with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product. The crude product was added into 2mol / L potassium hydroxide (30mL) and stirred for 2h. Dichloromethane (50 mL) was added, the layers were separated, the aqueous phase was acidified with 2 mol / L dilute hydrochloric acid, filtered, washed with water, and dried to obtain a white solid 24 (1.34 g), yield 72%. Melting point 179-180°C; 1 HNMR (400M...
Embodiment 3
[0059] Example 3: (E)-methyl 3-(7-benzo[1,2,3]thiadiazolyl)acrylate (30) and (E)-3-(2-hydroxy-1-naphthyl) Synthesis of Methyl Acrylate (31)
[0060]
[0061] Dissolve (E)-3-arylacrylic acid 22 or 23 (2mmol) in methanol (25mL), add concentrated sulfuric acid (0.72g) at room temperature, and heat to reflux for 8h. Cool to room temperature, add dichloromethane (50mL) and water (50mL), separate the layers, extract the aqueous phase with dichloromethane, combine the organic phases, wash the organic phase with sodium bicarbonate, dry over anhydrous sodium sulfate, filter, and concentrate to obtain product.
[0062] (E)-methyl 3-(7-benzo[1,2,3]thiadiazolyl)acrylate (30)
[0063] White solid, yield 94%. The melting point is 118-120°C. 1 HNMR (400MHz, CDCl 3 ): δ8.70(d, J=8.4Hz, 1H), 7.95(d, J=16.0Hz, 1H), 7.84(d, J=7.2Hz, 1H), 7.73(t, J=8.0Hz, 1H ), 6.46(d, J=16.0Hz, 1H), 3.88(s, 3H). 13 CNMR (100MHz, CDCl 3 ): δ166.7, 159.6, 142.3, 138.8, 131.5, 127.8, 125.9, 122.0, 77.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com