Dendritic polymer with POSS group and preparation method thereof
A dendritic and polymer technology, applied in the field of POSS base, can solve the problem that dendritic polymers have no relevant literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) Preparation of POSS-based intermediate NH 2 -POSS- (vinyl) 7
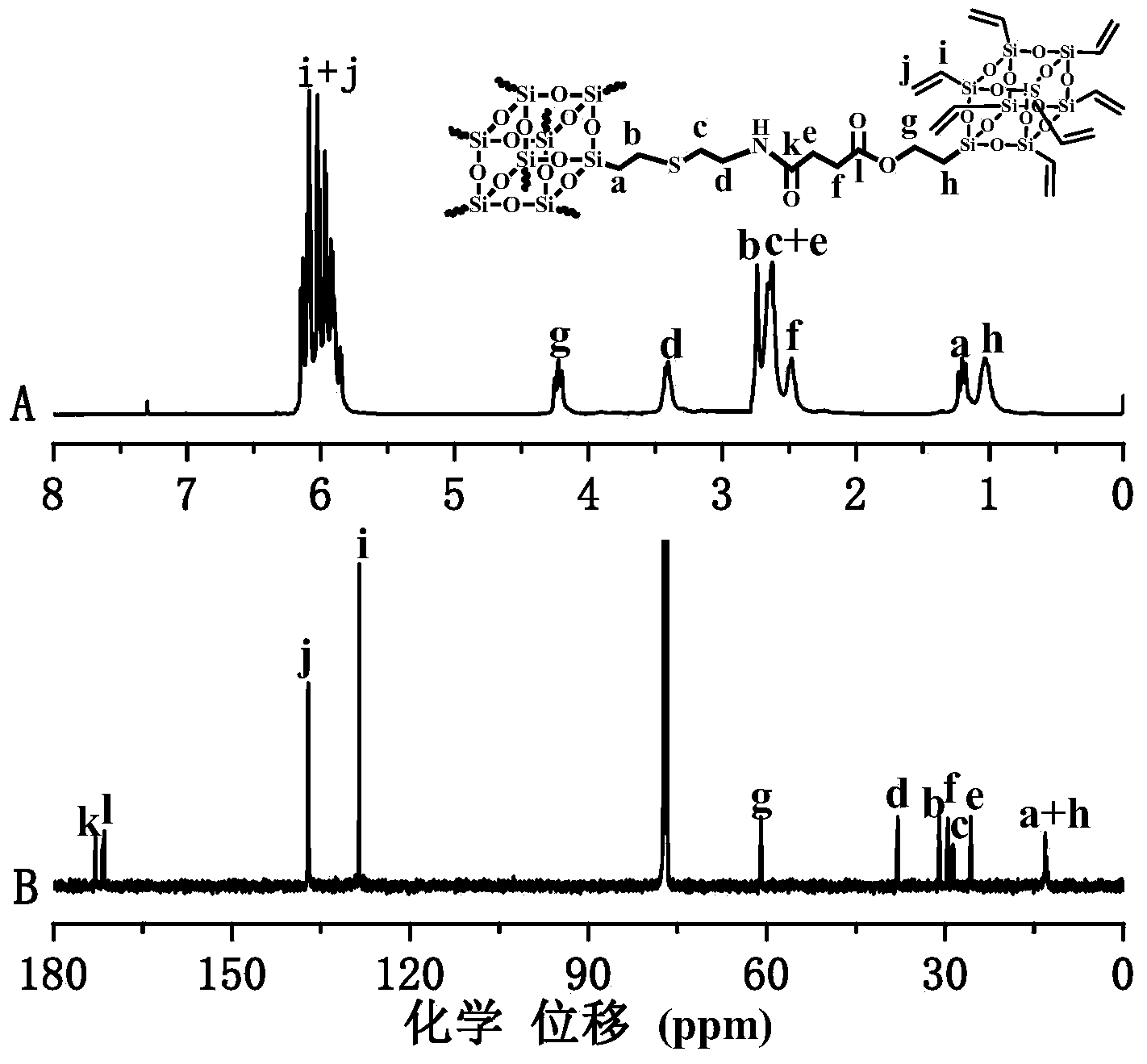
[0076] Put octavinyl cage oligomeric silsesquioxane (T8) produced by American Hybrid Company and cysteamine in dimethylformamide at a molar ratio of 1:1, and add 0.1 equivalent of 2,2-dimethyl Oxygen-2-phenylacetophenone (DMPA), ultraviolet radiation reacted in 2 hours, after the reaction was finished, the product obtained was precipitated in ether, then by silica gel column chromatography (with tetrahydrofuran and normal hexane volume ratio of The mixed solvent of 6:1 is the eluting agent, purified by silica gel (200-300 mesh), and after vacuum drying, the slightly yellow product can be obtained, which is the POSS-based intermediate NH 2 -POSS- (vinyl) 7 ;
[0077] (2) Preparation of dendrimers NH with POSS groups 2 -POSS 8 -(isobutyl) 49
[0078] The NH prepared in step (1) 2 -POSS- (vinyl) 7 SH-POSS-(isobutyl) produced by American Hybrid Company 7 Add 0.2 equivalents of 2,2-dimethoxy-2-phen...
Embodiment 2
[0081] (1) Preparation of POSS-based intermediate COOH-POSS-(vinyl) 7
[0082] Put octavinyl cage oligomeric silsesquioxane (T8) and mercaptopropionic acid in chloroform at a molar ratio of 1:0.8, and add 0.15 equivalents of 2,2-dimethoxy-2-phenyl Acetophenone (DMPA) was reacted by ultraviolet radiation for 3 hours. After the reaction was completed, the product obtained was precipitated in ether, and then by silica gel column chromatography (with THF and n-hexane volume ratio as a 1:1 mixed solvent was Eluent, silica gel (200-300 mesh)) purification, after vacuum drying, a slightly yellow product can be obtained, which is the POSS-based intermediate COOH-POSS-(vinyl) 7 ;
[0083] (2) Preparation of dendritic polymer COOH-POSS with POSS groups 8 -(cyclopentyl) 49
[0084] COOH-POSS-(vinyl) prepared in step (1) 7 SH-POSS-(cyclopentyl) produced by American Hybrid Company 7 Add 0.2 equivalents of azobisisobutyronitrile (AIBN) to tetrahydrofuran at a molar ratio of 1:7, heat...
Embodiment 3
[0088] (1) Preparation of POSS-based intermediate OH-POSS-(vinyl) 9
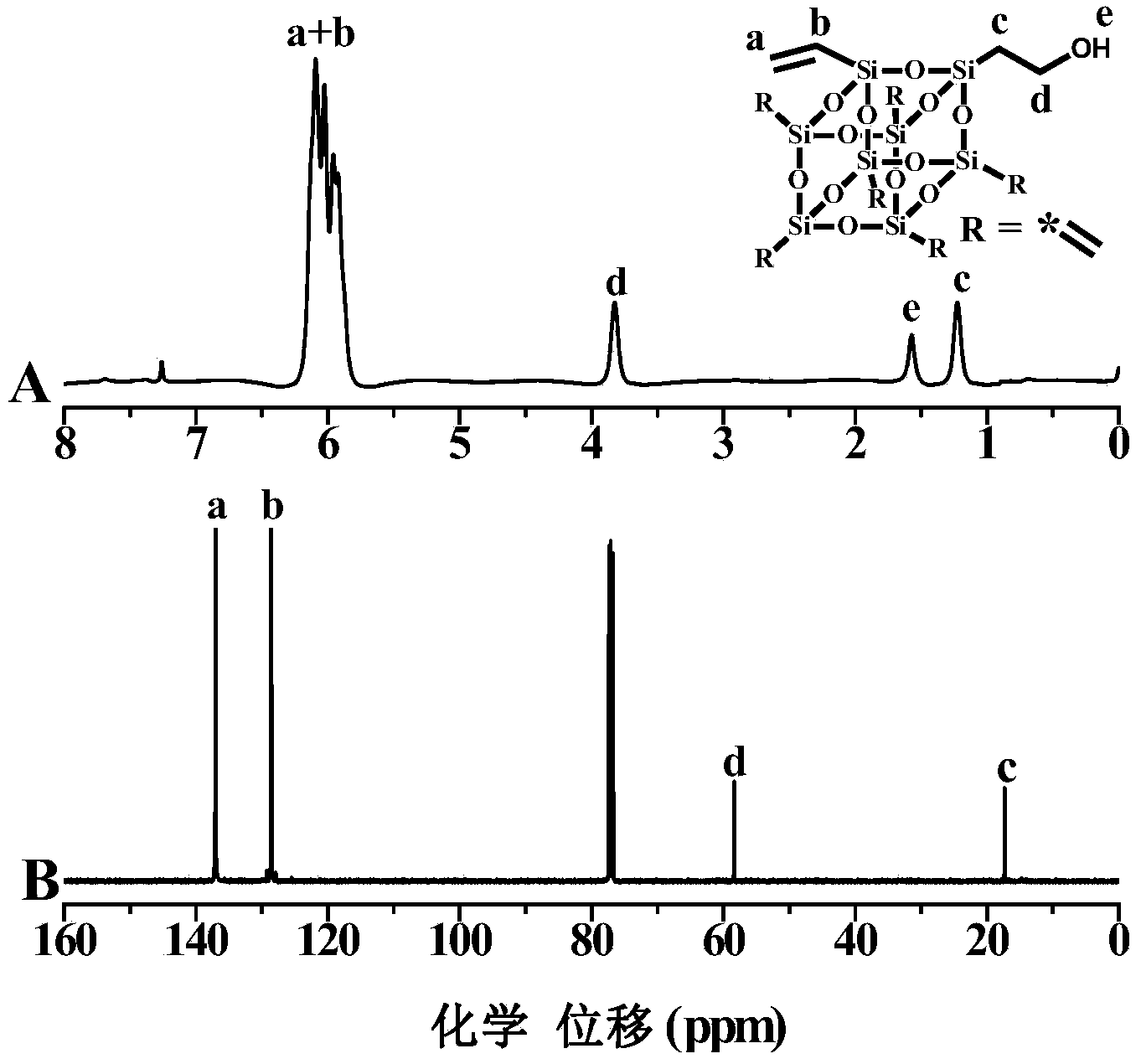
[0089] Decavinyl cage oligomeric silsesquioxane (T10) (synthesized according to J.Am.Chem.Soc.2013, 135, 12259-12269) and mercaptoethanol in dichloro In methane, add 0.3 equivalents of 2,2-dimethoxyl-2-phenylacetophenone (DMPA), and react with ultraviolet radiation for 3.5 hours. 1:1) in a mixed solvent, and then purified by silica gel column chromatography (dichloromethane and n-hexane volume ratio of 1:1 mixed solvent as eluent, silica gel (200-300 mesh)) purification, vacuum After drying, the slightly yellow product can be obtained, which is the POSS-based intermediate OH-POSS-(vinyl) 9 ;
[0090] (2) Preparation of dendritic polymer OH-POSS with POSS groups 10 -(isobutyl) 63
[0091] The OH-POSS-(vinyl) prepared in step (1) 9 With SH-POSS-(isobutyl) 7 Add 0.2 equivalents of azobisisobutyronitrile (AIBN) to chloroform at a molar ratio of 1:30, heat to 60°C and stir for 8 hours, then precipitate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com