Preparation method of dihydrooxazinyl naphthalene compound
A naphthodihydroxazine and compound technology, which is applied in the field of preparation of naphthodihydroxazine compounds, can solve the problems of short reaction time, troublesome handling, limited scope of application of substrates, etc. Effects of safety, easier storage and transportation, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 A method for preparing naphthodihydroxazine compounds, the method refers to adding catalyst aluminum powder, paraformaldehyde, aromatic amine compound aniline and β-naphthol to water in sequence, and proceeding at a temperature of 50°C Reaction, the whole reaction process is tracked by thin layer chromatography, that is, samples are taken every 5 minutes, and the reaction solution is dripped on the silica gel plate with a capillary tube, and the raw material solution β-naphthol and aniline are sampled. On the same straight line, put the silica gel plate into the exhibition bottle containing the mixture of petroleum ether and ethyl acetate with a volume ratio (mL / mL) of 30:1. After the plate movement is completed, put the silica gel plate under an ultraviolet lamp or an iodine bottle for observation. If there is no point flush with the raw material solution β-naphthol in the reaction solution, it indicates that the reaction is complete, and the reaction solu...
Embodiment 2
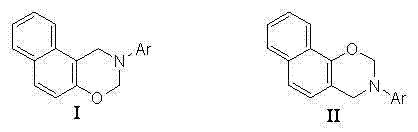
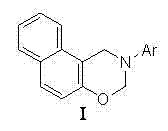
[0023] Example 2 A method for preparing naphthodihydroxazine compounds, the method refers to adding catalyst gallium powder, paraformaldehyde, 2-methylaniline and β-naphthol to water in sequence, and proceeding at a temperature of 40°C reaction, the whole reaction process is Example 1 Described thin-layer chromatography tracking, obtains reaction solution; This reaction solution presses Example 1 The method is sequentially extracted, dried, concentrated, and separated by column chromatography to obtain the naphthodihydroxazine compound of the product I. The product structural formula is as follows (Ar=2-methylphenyl in the structural formula):
[0024]
[0025] Among them: the molar ratio of gallium powder to β-naphthol is 1:20, the molar ratio of β-naphthol to paraformaldehyde is 1:2, and the molar ratio of β-naphthol to 2-methylaniline is 1:1.3 , The molar ratio of β-naphthol to water is 1:2000.
[0026] The reaction time was 1 h, and the yield was 85%.
Embodiment 3
[0027] Example 3 A method for preparing naphthodihydroxazine compounds, the method refers to adding catalyst nickel powder, paraformaldehyde, 4-methylaniline and β-naphthol to water in sequence, and proceeding at a temperature of 50°C reaction, the whole reaction process is Example 1 Described thin-layer chromatography tracking, obtains reaction solution; This reaction solution presses Example 1 The method is sequentially extracted, dried, concentrated, and separated by column chromatography to obtain the naphthodihydroxazine compound of the product I. The product structural formula is as follows (Ar=4-methylphenyl in the structural formula):
[0028]
[0029] Among them: the molar ratio of nickel powder to β-naphthol is 1:10, the molar ratio of β-naphthol to paraformaldehyde is 1:2, and the molar ratio of β-naphthol to 4-methylaniline is 1:1 , The molar ratio of β-naphthol to water is 1:800.
[0030] The reaction time was 0.8 h, and the yield was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com