Preparation method of cefditoren pivoxil
A technology for cefditoren pivoxil and cephem, which is applied in the field of preparation of cephalosporins, can solve the problems of poor yield, strong corrosiveness of reagents and the like, and achieves the effects of improved yield, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
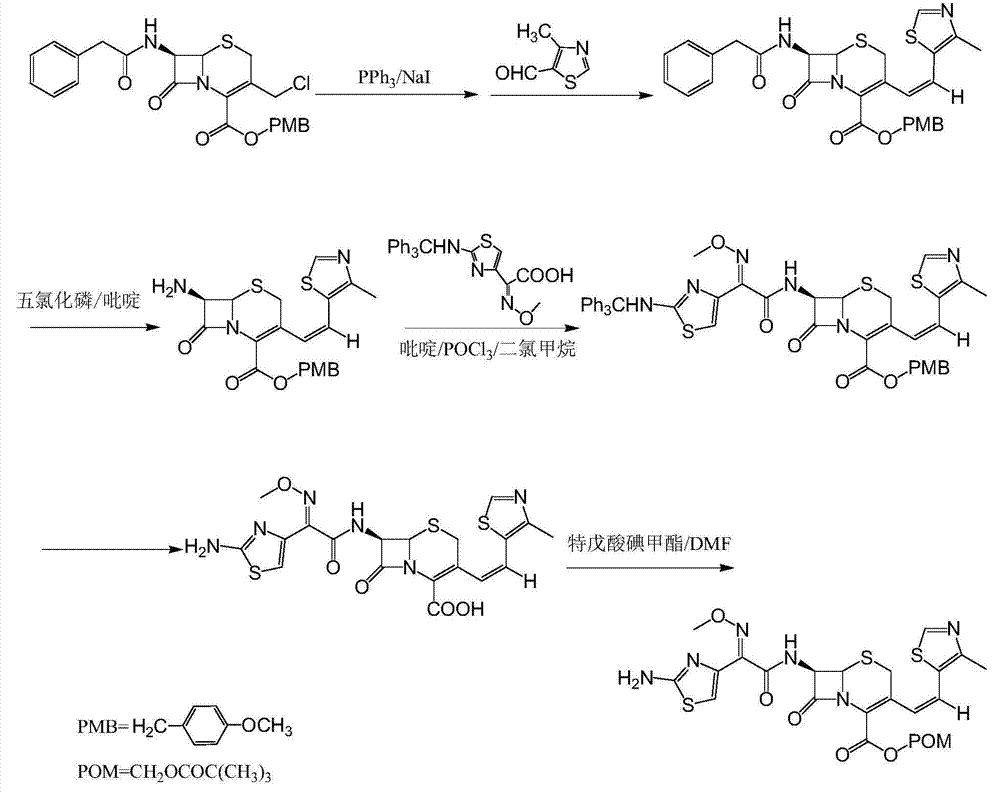
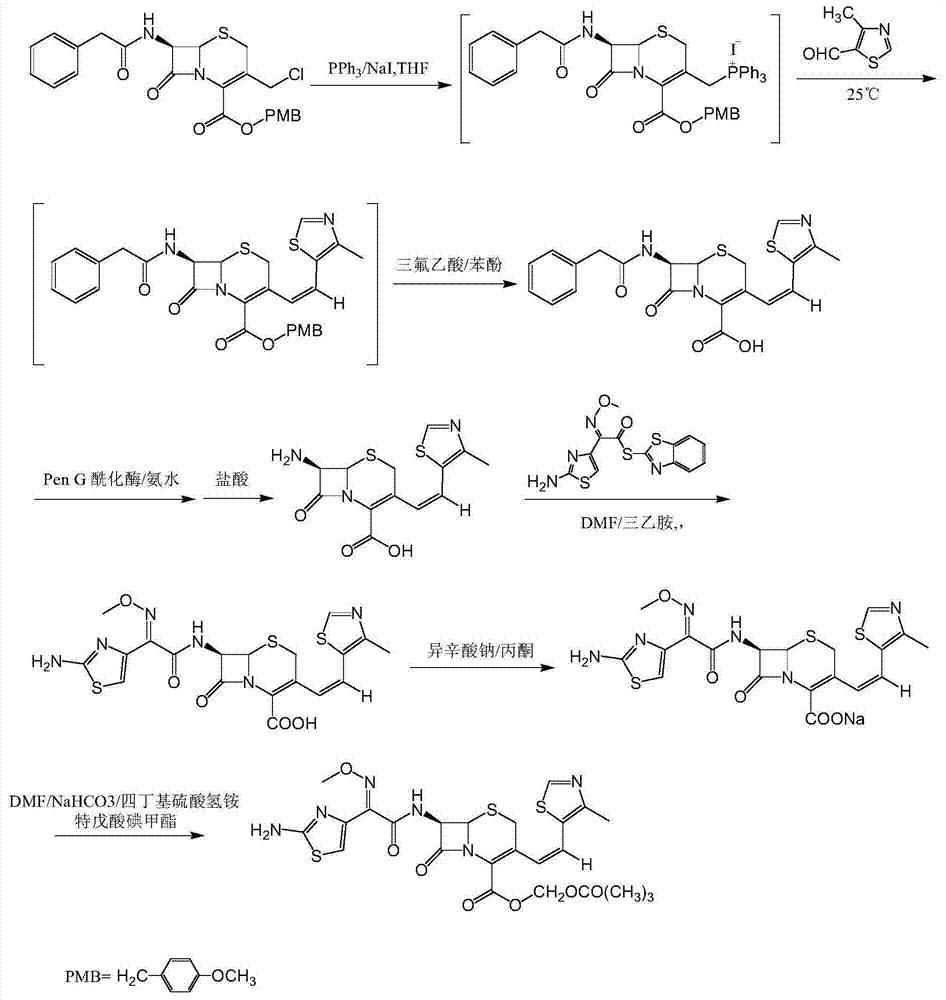
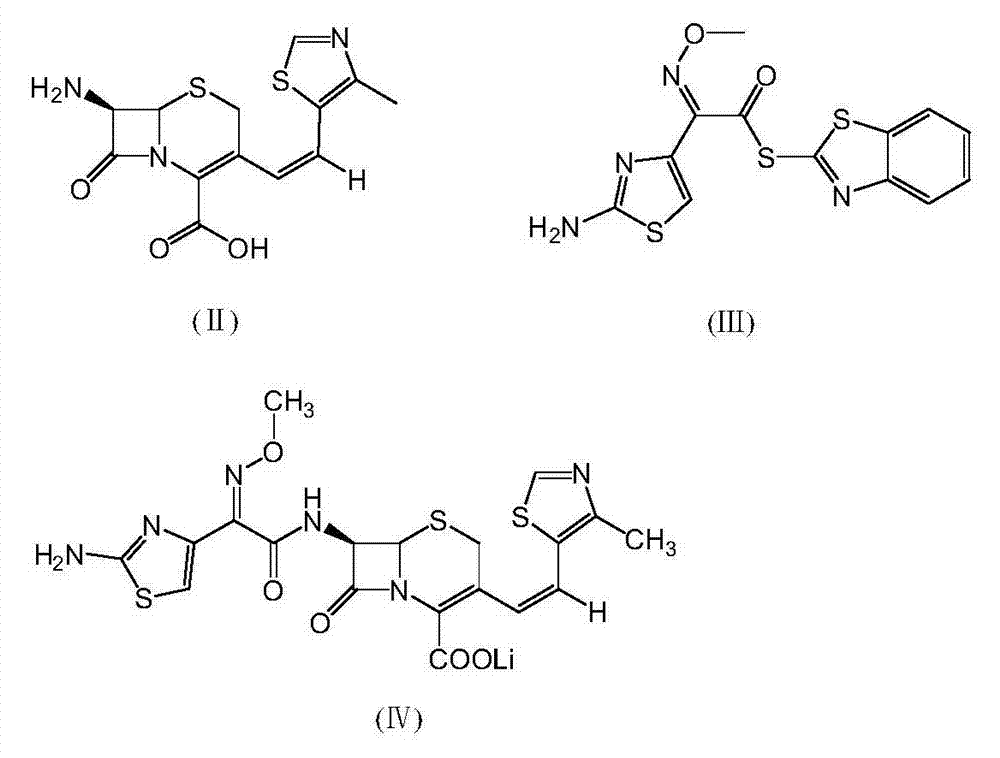
[0026] (6R,7R)-7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido]-3-[(Z)-2-(4-methyl Preparation of lithium-5-thiazolyl)vinyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
[0027] Put 200g of 7-amino-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-3-cephem-4-carboxylic acid, 2-methoxyimino into the reaction flask - 240g of 2-(2-amino-4-thiazolyl)-(z)-benzothiazolyl thioacetate, 2000ml of dichloromethane, lower the temperature to 0°C, and add 120g of ethylamine dropwise. Add water, separate the layers, adjust the pH of the aqueous layer to 2.8 with dilute hydrochloric acid, and centrifuge until dry. The filter cake was completely dissolved with 5000ml of acetone, 130g of lithium tert-butoxide was added to react for 1 hour, filtered, and the product was dried to obtain 270g of the title compound with a yield of 80.0%.
Embodiment 2
[0029] Put 200g of 7-amino-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-3-cephem-4-carboxylic acid, 2-methoxyimino into the reaction flask - 240 g of 2-(2-amino-4-thiazolyl)-(z)-benzothiazolyl thioacetate, 2000 ml of dichloromethane, lower the temperature to 0°C, and add 145 g of triethylamine dropwise. Add water, separate the layers, adjust the pH of the aqueous layer to 2.8 with dilute hydrochloric acid, and centrifuge until dry. The filter cake was completely dissolved with 5000ml of acetone, 130g of lithium tert-butoxide was added to react for 1 hour, filtered, and the product was dried to obtain 276g of the title compound with a yield of 82.1%.
Embodiment 3
[0031] Put 200g of 7-amino-3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-3-cephem-4-carboxylic acid, 2-methoxyimino into the reaction flask - 240 g of 2-(2-amino-4-thiazolyl)-(z)-benzothiazolyl thioacetate, 2000 ml of dichloromethane, lower the temperature to 0°C, and add 157 g of triethanolamine dropwise. Add water, separate the layers, adjust the pH of the aqueous layer to 2.8 with dilute hydrochloric acid, and centrifuge until dry. The filter cake was completely dissolved with 5000ml of acetone, 130g of lithium tert-butoxide was added to react for 1 hour, filtered, and the product was dried to obtain 254g of the title compound with a yield of 74.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com