Preparation and anti-tumor application of natural active drug-polysaccharide targeted compound
A technology for targeting complexes and active drugs, which is applied in the field of preparation of natural active drug-polysaccharide targeting complexes, can solve the problems of limited application of preparations, insoluble in water, low bioavailability, etc., and achieves improved antitumor activity. , good dispersion, smooth surface effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
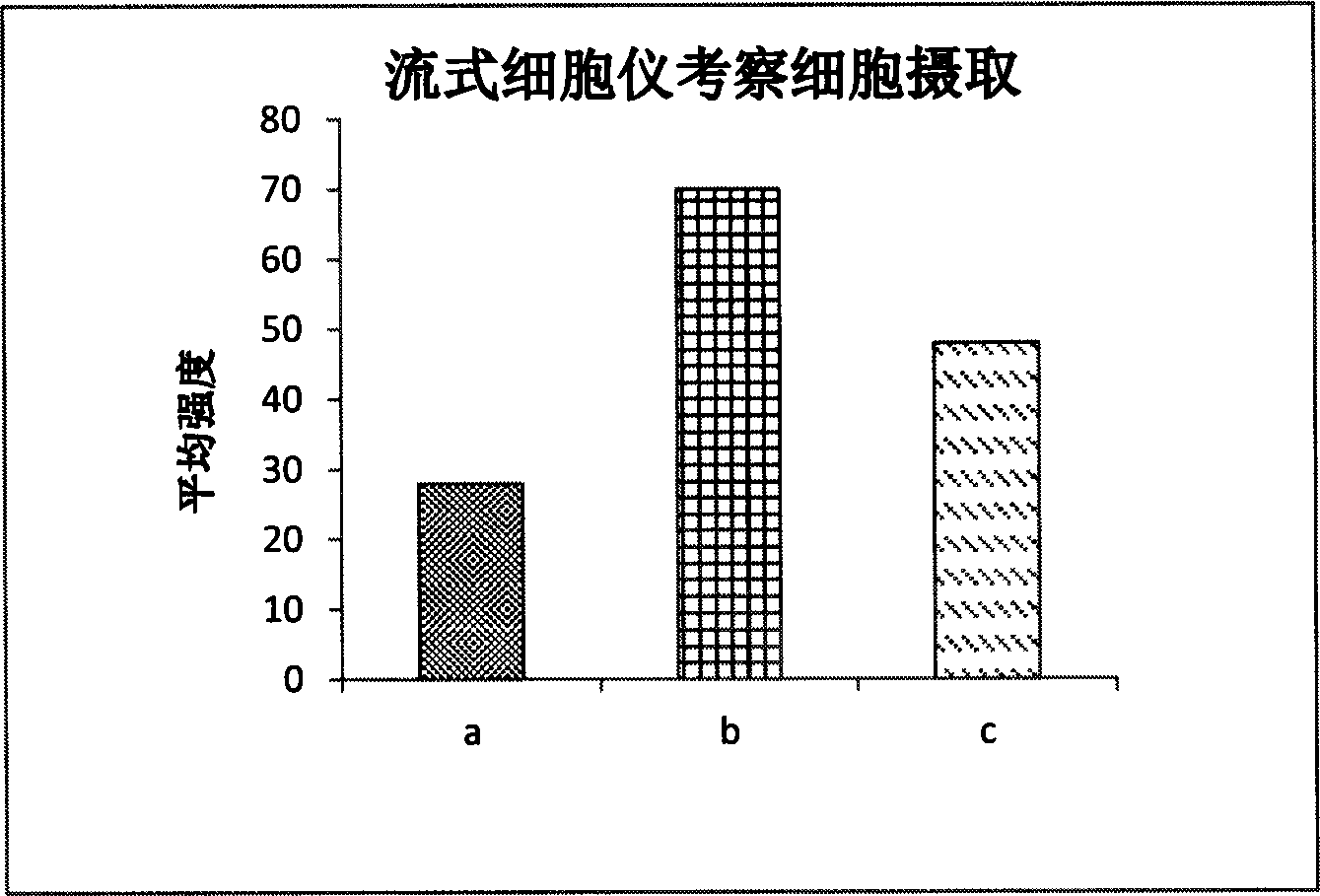
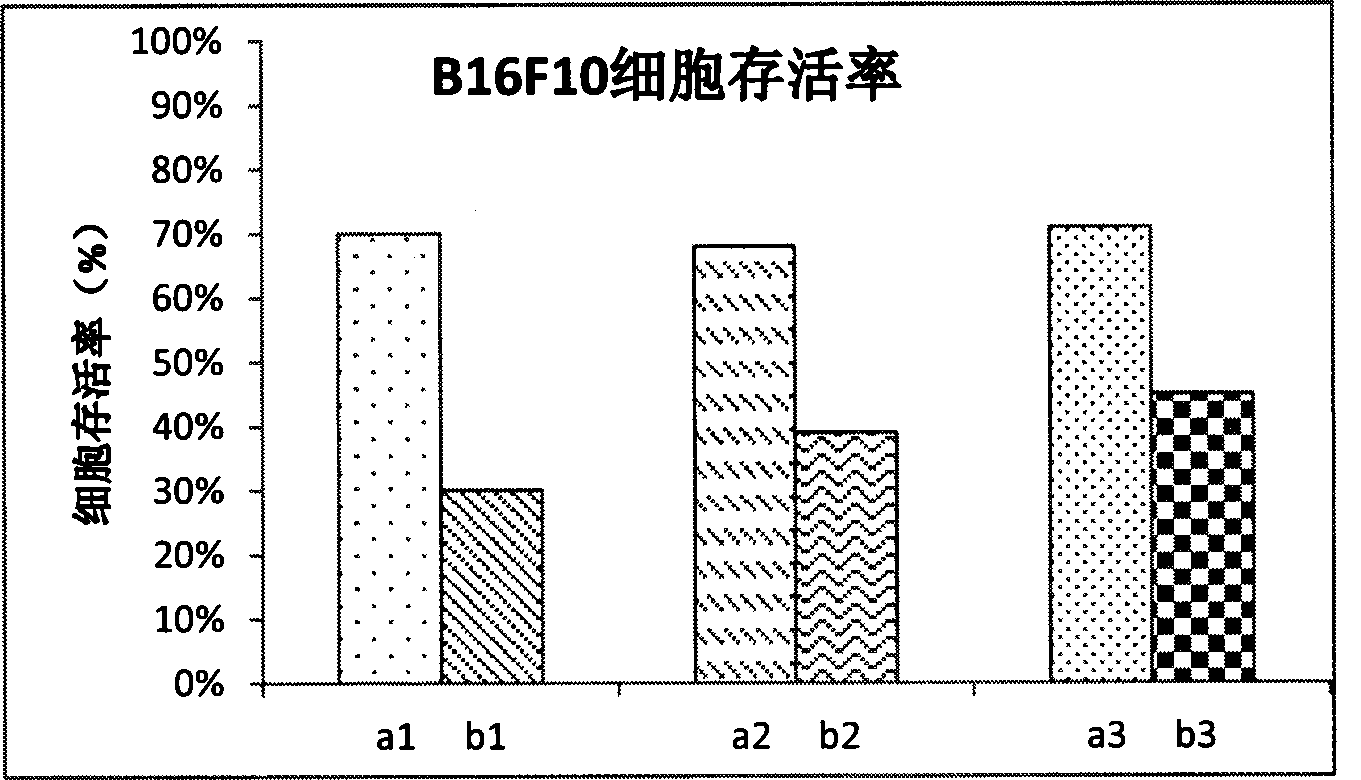
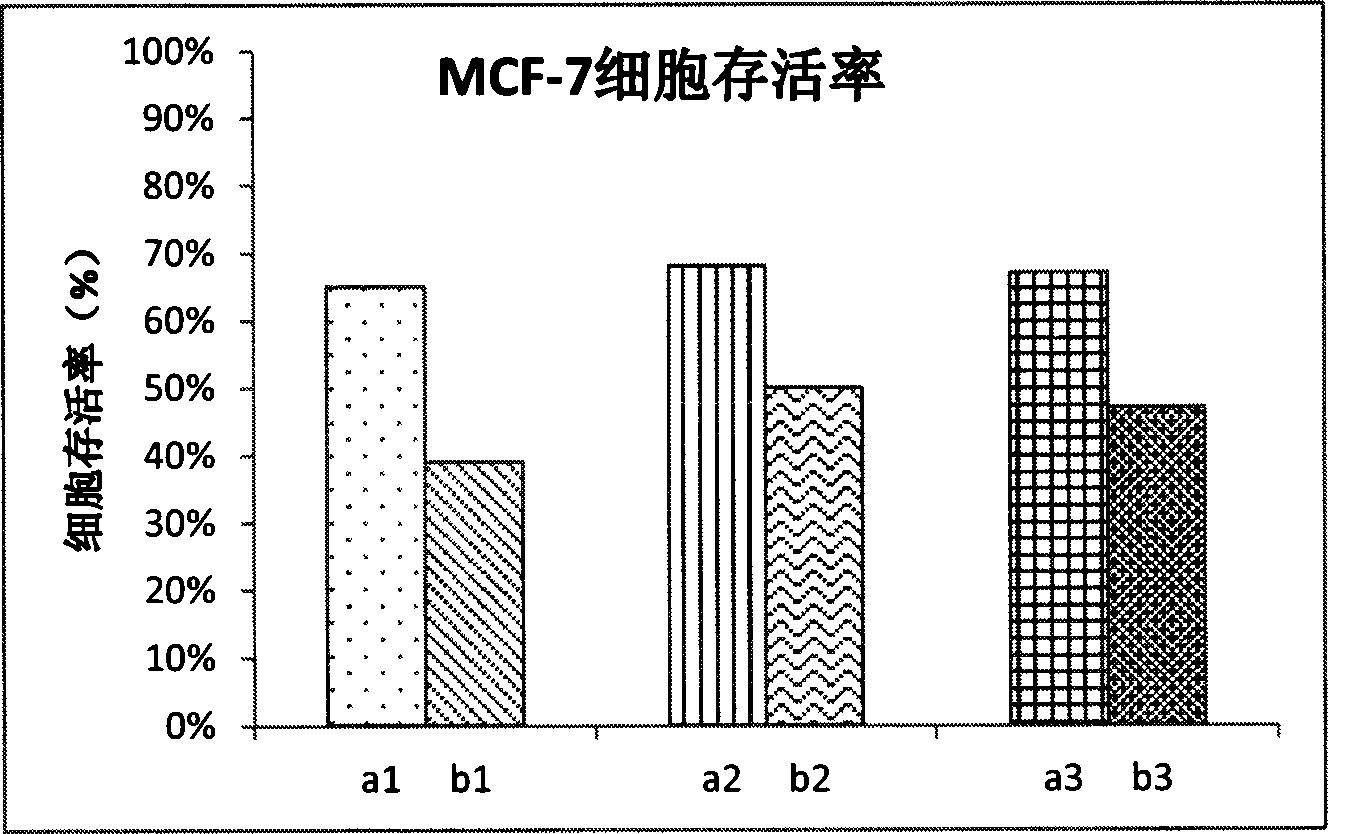
Image
Examples
Embodiment 1
[0094] Embodiment 1: Preparation of curcumin-carboxymethyl dextran targeting complex
[0095] Take 0.2mol of carboxymethyl dextran and add it to 15ml of water, stir magnetically until it is completely dissolved, adjust the pH to about 6-7 with acetic acid, then add 0.6mol of propylenediamine, mix well and then add 0.2mol of 1-ethyl-(3 - Dimethylaminopropyl) carbodiimide (EDC) and 0.3mol hydroxysuccinimide (NHS), react at room temperature for 12 hours, dialyze for 2 days, and freeze-dry to obtain a carboxymethyl dextran intermediate with free one-terminal amino group. Take the above-mentioned intermediate and disperse it in an appropriate amount of methanol, add 0.9 mol of curcumin in methanol solution, ultrasonically reflux for 2.5 hours, wash the crude product repeatedly with anhydrous methanol, anhydrous ether, and dichloromethane until the filtrate is colorless, and vacuum-dry to obtain Purified curcumin-carboxymethyl dextran amphiphilic conjugate. 4 mg of curcumin-carboxy...
Embodiment 2
[0096] Embodiment 2: Preparation of ursolic acid-low molecular weight heparin targeting complex
[0097] 300mg of ursolic acid was dissolved in dichloromethane, and gradually dropped into the dichloromethane solution in which 280mg of pyridinium chlorochromate was dissolved at 10°C. After the dropwise addition was completed, stir at room temperature for 1.5h. The reaction solution was filtered, washed, washed with water, dried, filtered and concentrated, followed by silica gel column chromatography, eluted and concentrated to obtain a white solid. Take 59 mg of the white solid and 80 mg of hydroxylamine hydrochloride and dissolve it in 7 ml of pyridine, connect it to a drying tube, and reflux at 115°C for about 6 hours. After the reaction solution was cooled to room temperature, it was washed with distilled water, filtered, washed with water, and dried to obtain a white solid. Take this white solid 120mg, 1.05g sodium cyanoborohydride, 1.05g sodium acetate, dissolve in metha...
Embodiment 3
[0098] Example 3: Preparation of gambogic acid-hyaluronic acid targeting complex
[0099] Weigh 0.6mol of gambogic acid (GA) and place it in an eggplant-shaped bottle, add 30mL of tetrahydrofuran, ice bath, 2 Under the condition, add dicyclohexylcarbodiimide (DCC) and hydroxysuccinimide (NHS) successively, the molar ratio of GA, DCC, NHS is 1: 1.5: 1.2 successively. After reacting in ice bath for 30 min, move to room temperature for 24 h. The precipitate DCU was removed by vacuum filtration to obtain a filtrate. The filtrate was precipitated with 3 times the amount of n-hexane for 12 h, filtered, and dried in vacuo to obtain succinimidyl GA. Weigh succinimide-based GA and dissolve it in N,N-dimethylformamide, slowly add it dropwise to 2mL cystamine, and drop it over 55min. React for another 8 hours, precipitate with saturated saline, and filter to obtain aminated GA as a yellow precipitate, which is sequentially washed with acid, washed with water, dried in vacuum, and stor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com