Baicalin injection with anti-influenza virus effect
An anti-influenza virus and glycoside injection technology, applied in the field of baicalin injection, can solve the problems of high price, low oral availability, small distribution volume, etc., and achieve the effect of rapid onset of action, high bioavailability, and prolonged survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
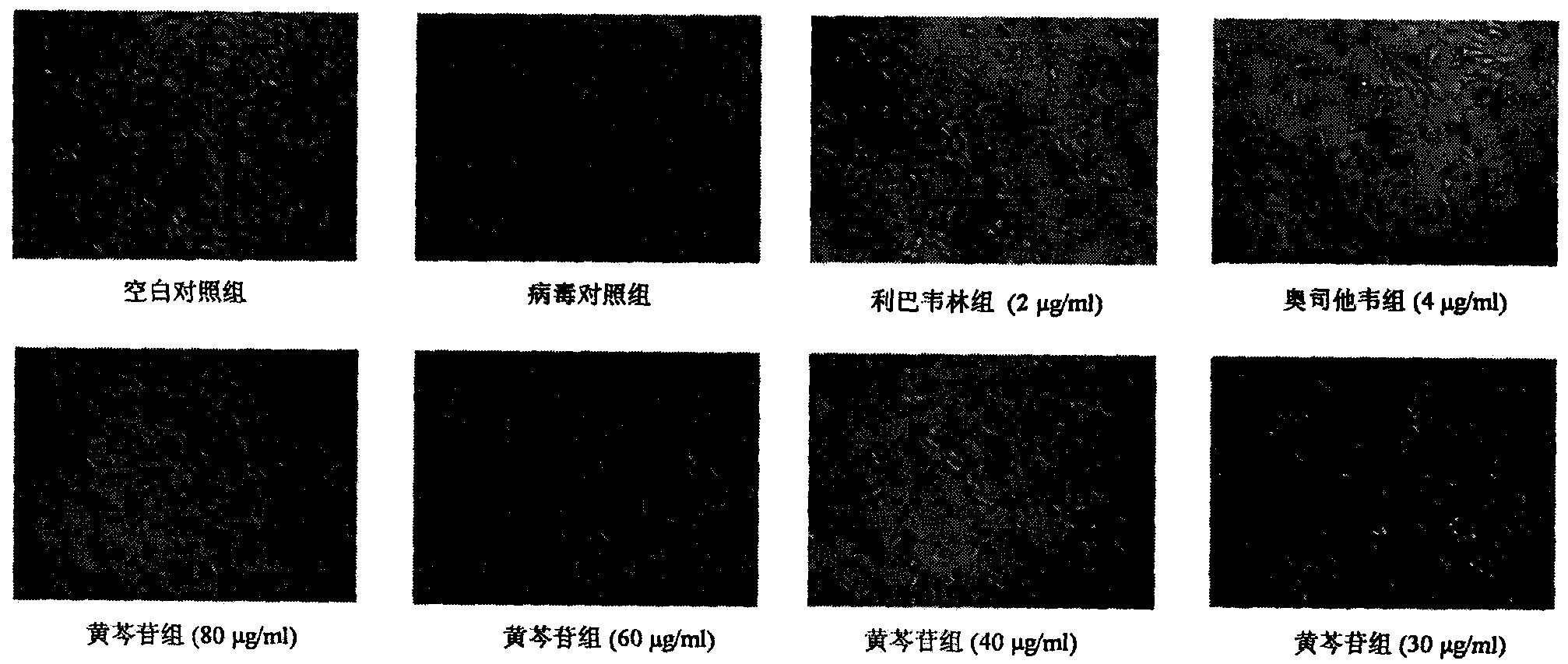
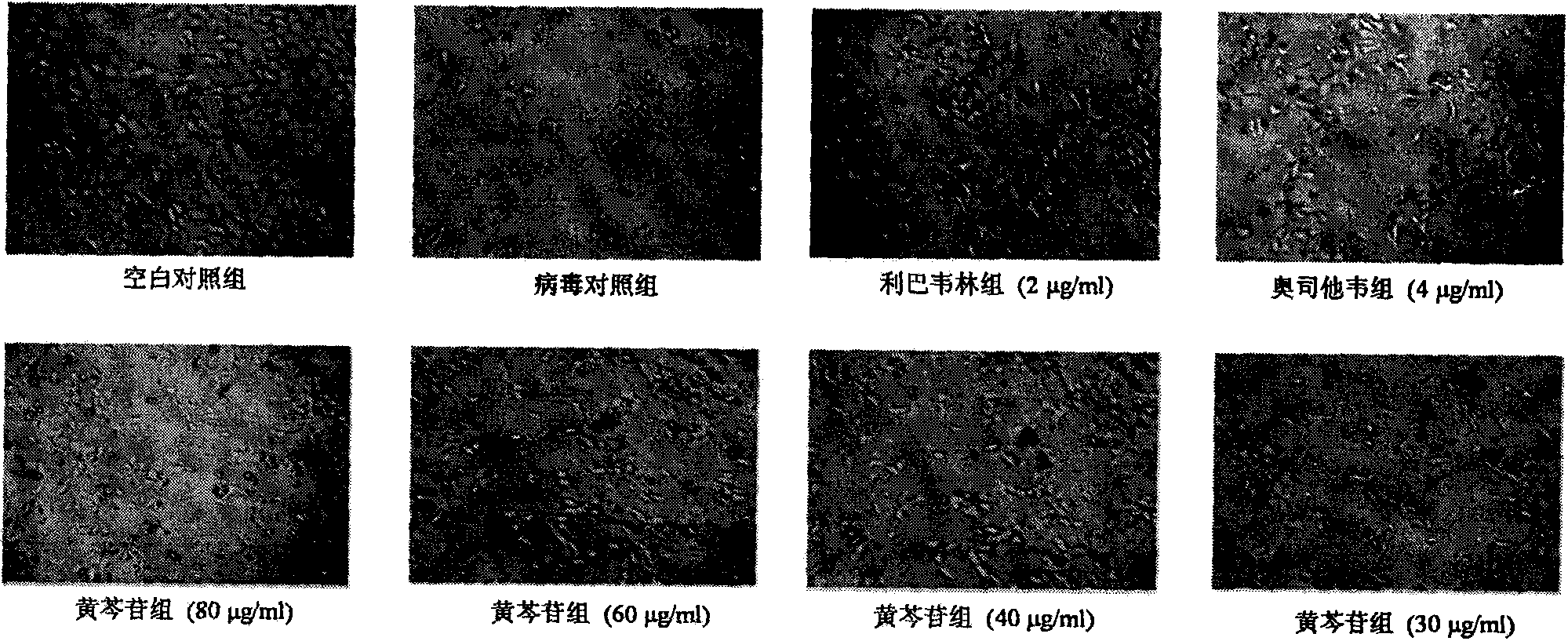
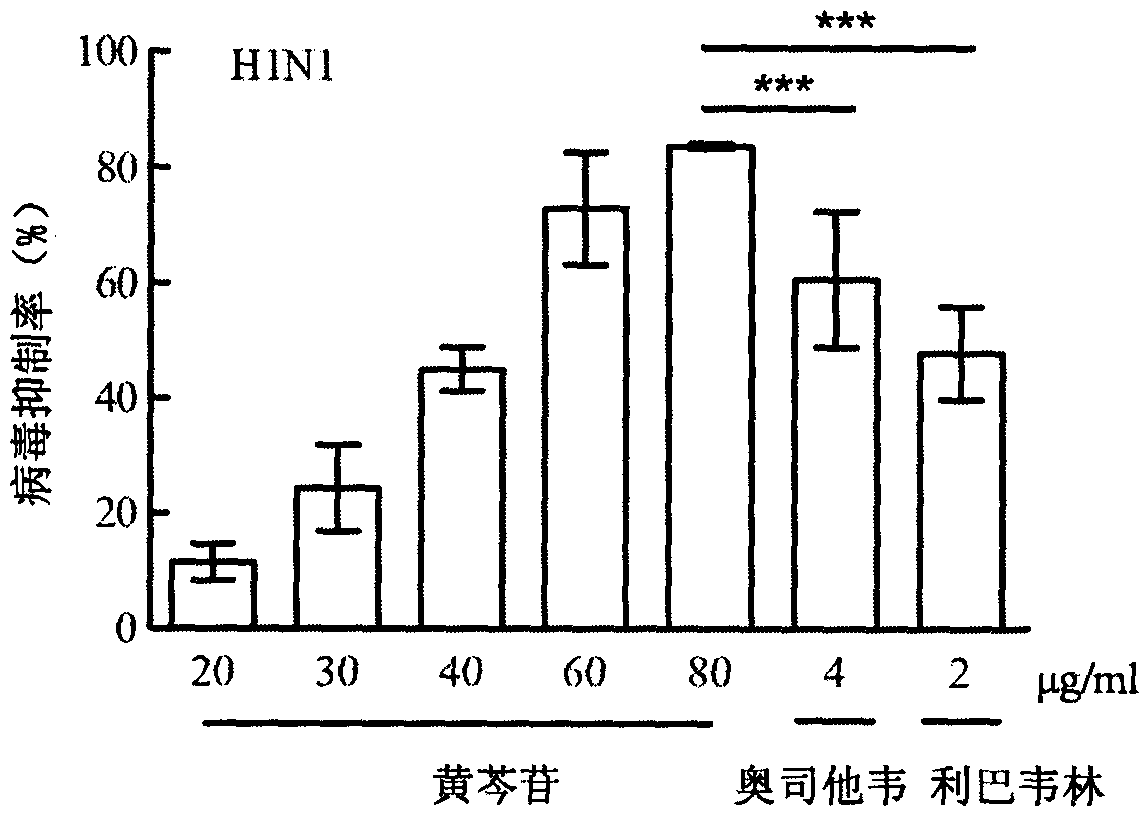
[0019] Inhibitory effect of baicalin injection on MDCK cells infected by influenza A virus A / FM / 1 / 47(H1N1) and A / Beijing / 32 / 92(H3N2) and the effect on cell survival rate
[0020] Experimental settings Normal control group, virus control group, positive drug oseltamivir 4 μg / ml, ribavirin group 2 μg / ml and baicalin group 80 μg / ml, 60 μg / ml, 40 μg / ml, 30 μg / ml, 20 μg / ml ml. MDCK cells by 5×10 4 Inoculate a 96-well culture plate at a concentration of / ml, 100 μl per well, and store at 37°C in 5% CO 2 Cultured in the incubator for 24h to form a cell monolayer. After discarding the supernatant, add 100TCID 50 Infection cells with influenza virus A / FM1 / 1 / 47 (H1N1) or A / Beijing / 32 / 92 (H3N2), incubated at 37°C for 2 hours, removed the virus liquid, and added different concentrations of baicalin 80 μg / ml, 60 μg / ml ml, 40 μg / ml, 30 μg / ml, 20 μg / ml and positive control drug ribavirin group 2 μg / ml, oseltamivir group 4 μg / ml maintenance medium, normal control group and virus control g...
Embodiment 2
[0025] Effect of Baicalin Injection on the Death Protection Rate of Influenza A Virus A / FM / 1 / 47(H1N1) Infected Mice
[0026] The experiment used 17-20g ICR mice, which were randomly divided into 6 groups, which were set as normal control group, virus control group, oseltamivir 100mg / kg / d, ribavirin 100mg / kg / d and baicalin Low-dose group 50mg / kg / d, medium-dose baicalin 100mg / kg / d, high-dose baicalin group 200mg / kg / d. After 7 days of adaptive culture, the experiment was started. Except for the normal control group, mice in other groups were lightly anesthetized with ether, and inoculated into the nasal cavity equivalent to 8LD 50The allantoic fluid of chicken embryos containing influenza virus was 50 μl per chick, and the oseltamivir group was administered intragastrically 1 hour after infection, once a day, 0.2ml once a day, for a total of 5 days. The ribavirin group and the baicalin administration group were administered through the tail vein 1 hour after infection, and then...
Embodiment 3
[0033] Protective Effect of Baicalin Injection on Body Weight of Influenza A Virus A / FM / 1 / 47(H1N1) Infected Mice
[0034] After the virus infected mice, it caused viral pneumonia in mice, resulting in poor breathing, reduced food intake, and weight loss in mice. Weight protection is a useful apparent indicator for evaluating the inhibition of drugs on virus proliferation in vivo. Experimental results ( Figure 5 ) showed that, except for the mice in the normal group, the mice in the other groups began to lose weight and disease symptoms on the 3rd day, manifested as shrugging hair, eating poorly, becoming thin, curled up to avoid the cold, etc. The body weight of mice in the virus group decreased the most. Since baicalin, ribavirin and oseltamivir inhibited the proliferation of the virus in the lungs of mice, the body weight of mice in each administration group decreased more than that of the virus group. Small, all showed the effect of protecting the body weight of mice to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com