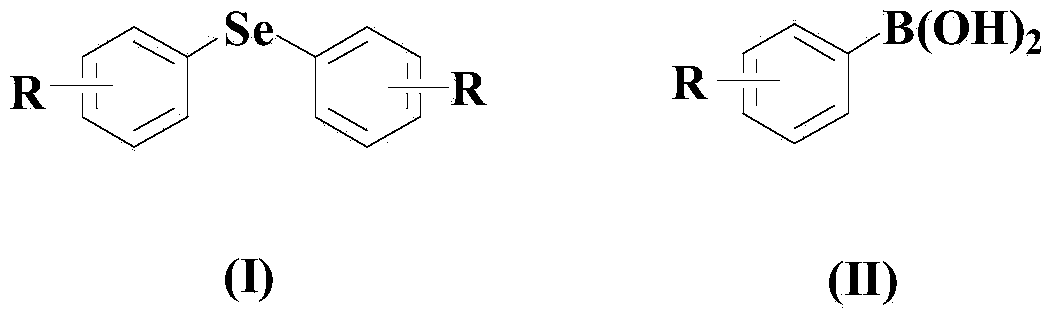
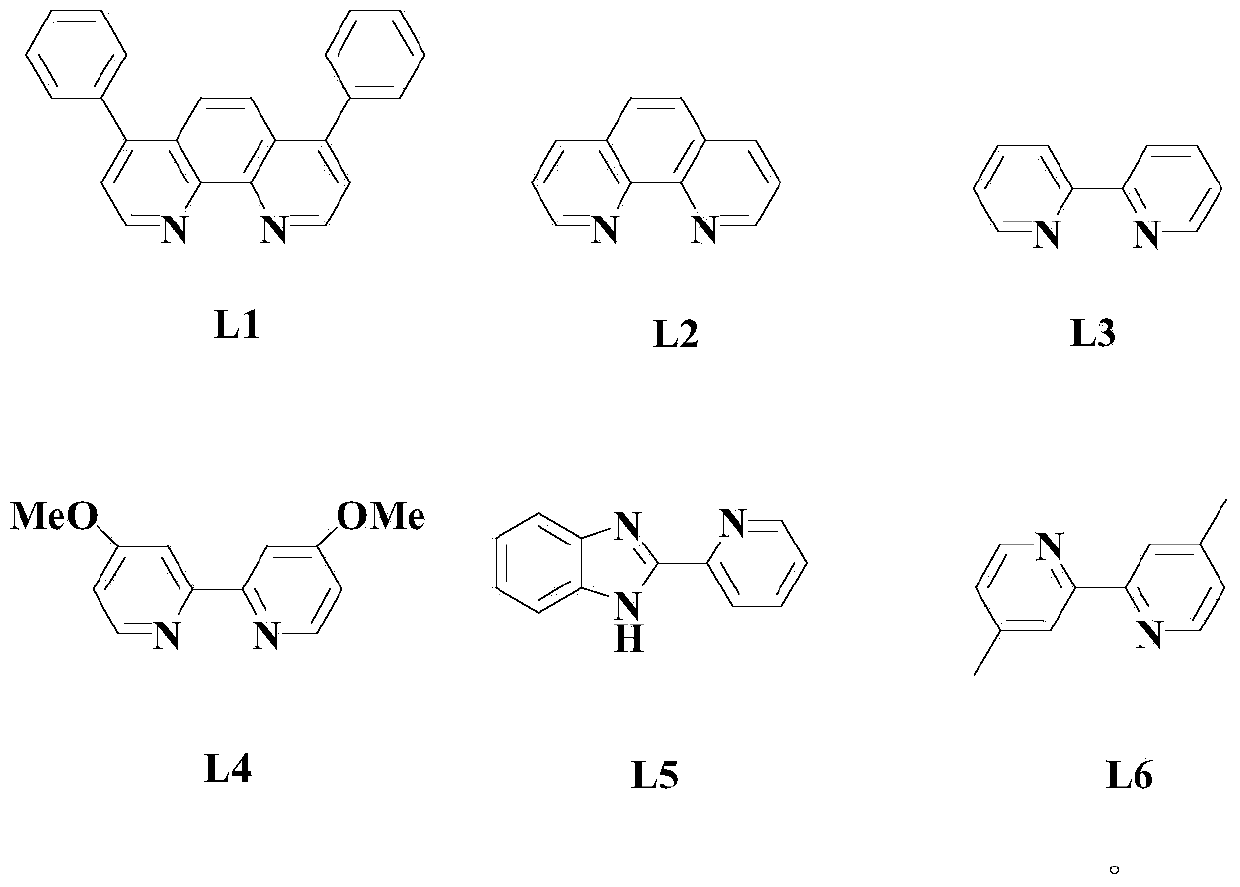
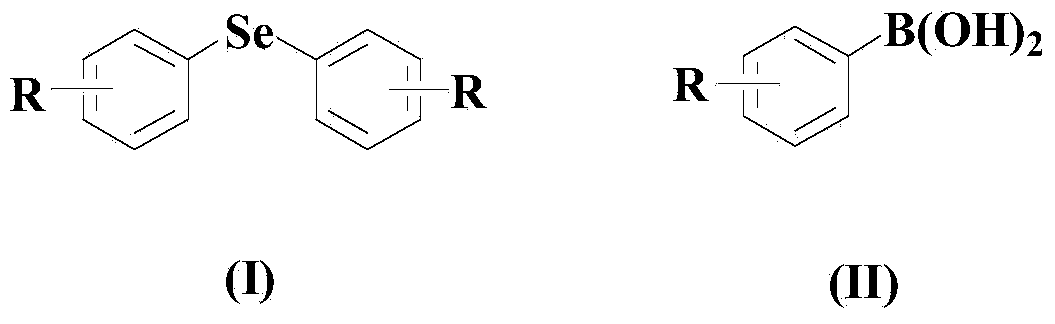
Aryl elemental selenium compound synthesis method
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of side reaction functional group compatibility, deterioration, unsatisfactory atom utilization, etc., and achieve simple reaction, high product yield and purity, good research value and The effect of industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthesis of diphenyl selenide
[0049]
[0050] In a dry and clean reactor, add 50ml solvent THF, then add 10mmol elemental selenium, 10mmol (II) compound, 0.5mmol CuCl, 0.5mmol organic ligand L1, and then add 10mmol di-tert-butyl peroxide. The reaction system was stirred and reacted at 50° C. for 30 hours under an air atmosphere.
[0051] After the reaction was finished, the reaction system was cooled to room temperature, and then the solvent was removed by rotary evaporation with a rotary evaporator to remove the solvent from the mixture obtained after the reaction, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product. The yield was It is 92.5%, and the purity is 98.9% (HPLC).
[0052] NMR: 1 H NMR (CDCl 3 ,500MHz): δ7.49(d,J=5Hz,4H),7.27-7.29(m,6H);
[0053] 13 C NMR (CDCl 3 ,125MHz): δ133.0(4C), 131.1(2C), 129.3(4C), 127.3(2C).
Embodiment 2
[0054] Embodiment 2: the synthesis of two (3-tolyl) selenides
[0055]
[0056] In a dry and clean reactor, add 50ml solvent 2-methyltetrahydrofuran, then add 10mmol elemental selenium, 20mmol (II) compound, 1mmol CuCl, 1mmol organic ligand L1, and then add 20mmol di-tert-butyl peroxide , the reaction system was stirred and reacted at 60° C. for 25 hours under an air atmosphere.
[0057] After the reaction was finished, the reaction system was cooled to room temperature, and then the solvent was removed by rotary evaporation with a rotary evaporator to remove the solvent from the mixture obtained after the reaction, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product. The yield was It was 73.8%, and the purity was 99.1% (HPLC).
[0058] NMR: 1 H NMR (DMSO-d 6 ,500MHz):δ7.42(s,2H),7.35(d,J=5Hz,4H),7.23-7.27(m,2H),2.39(s,6H);
[0059] 13 C NMR (CDCl 3 ,125MHz): δ138.8(2C), 132.7(2C), 130.9(2C), 130.0(2C), 128.0(2C),...
Embodiment 3
[0060] Embodiment 3: the synthesis of two (3-nitrophenyl) selenides
[0061]
[0062] In a dry and clean reactor, add 50ml solvent DMF, then add 10mmol elemental selenium, 30mmol (II) compound, 1.5mmol CuCl, 1.5mmol organic ligand L1, and then add 30mmol di-tert-butyl peroxide. The reaction system was stirred and reacted at 70° C. for 20 hours under an air atmosphere.
[0063] After the reaction was finished, the reaction system was cooled to room temperature, and then the solvent was removed by rotary evaporation with a rotary evaporator to remove the solvent from the mixture obtained after the reaction, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product. The yield was It is 81.8%, and the purity is 98.6% (HPLC).
[0064] NMR: 1 H NMR (CDCl 3 ,500MHz):δ8.32(s,2H),8.16(d,J=10Hz,2H),7.78(d,J=10Hz,2H),7.45-7.51(m,2H);
[0065] 13 C NMR (CDCl 3 ,125MHz): δ148.7(2C), 138.9(2C), 131.7(2C), 130.4(2C), 127.7(2C), 123.1(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com