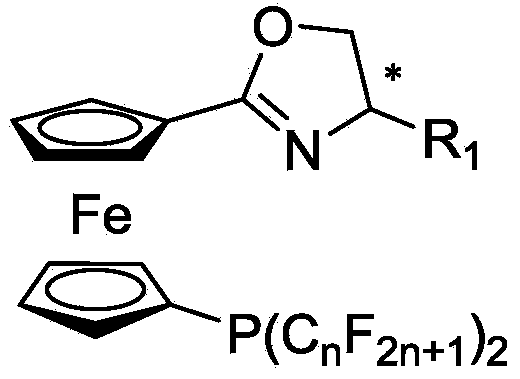
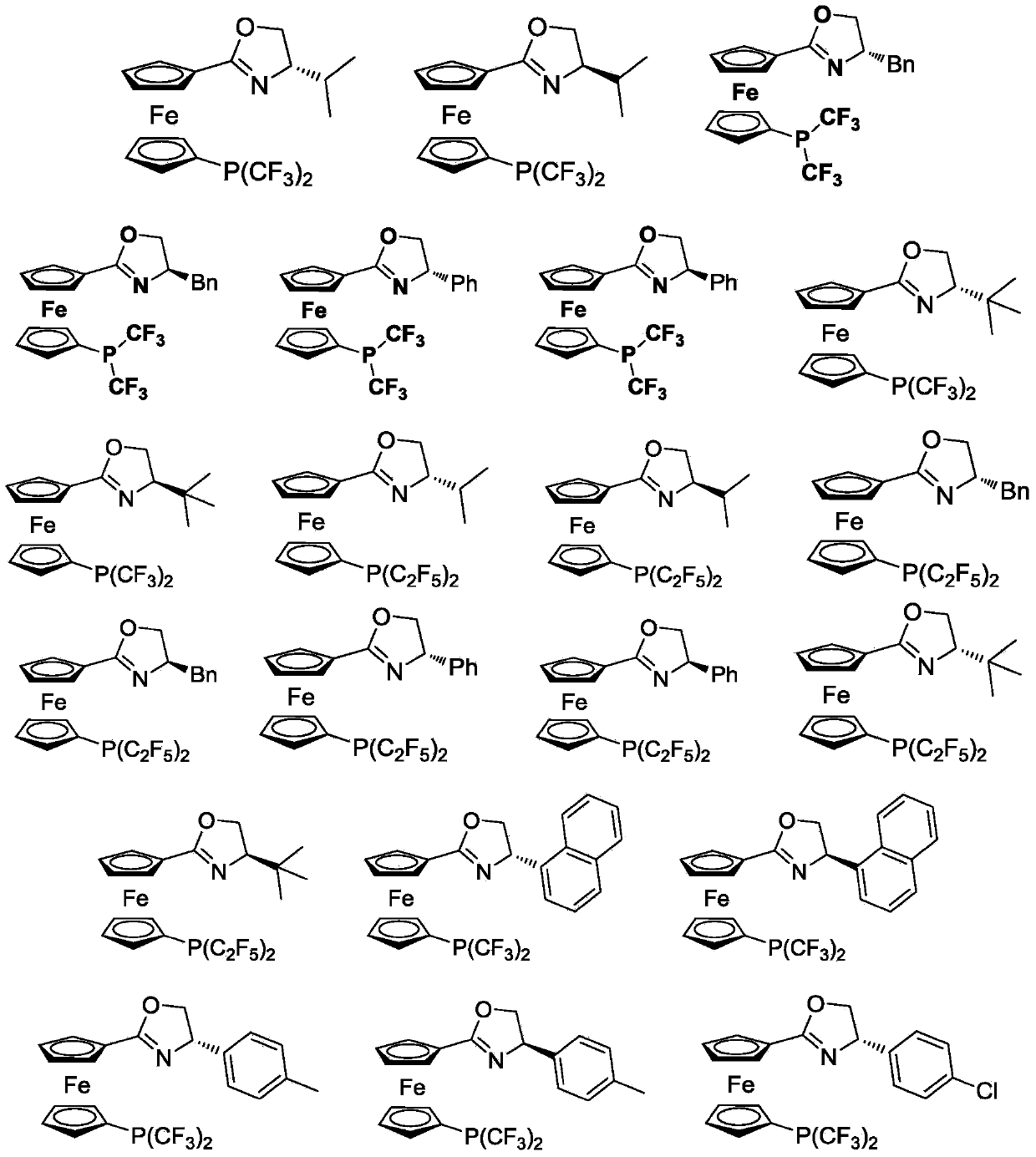
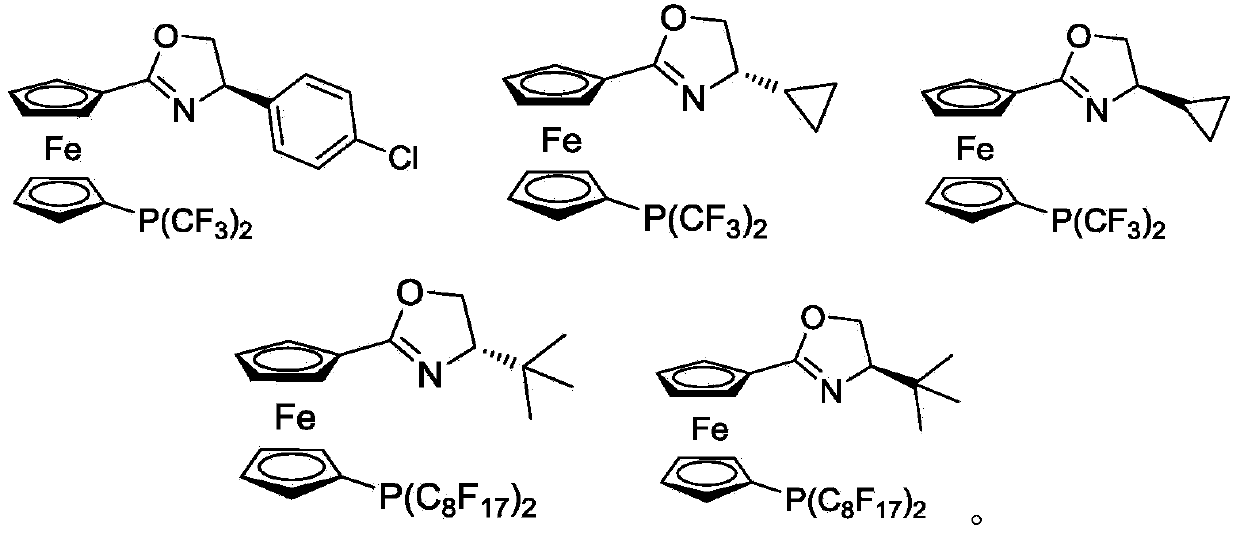
1, 1'-ferrocene perfluoroalkyl phosphine nitrogen ligand as well as preparation method and application thereof
A perfluoroalkylphosphine and ferrocene technology, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as poor results, complicated synthesis methods, and no regioselectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] In Example 1, the preparation method and conditions of the compound without specific experimental steps are the same as the preparation P1: (S)-1-(4,5-dihydro-4-isopropyl-oxazoline)-1'- The method and conditions of bistrifluoromethylphosphinoferrocene differ only in that different reaction substrates are used to prepare corresponding products.
[0079] P1: Synthesis of (S)-1-(4,5-dihydro-4-isopropyl-oxazoline)-1’-bistrifluoromethylphosphinoferrocene
[0080]
[0081] where rt means room temperature.
[0082] Under nitrogen protection, oxazoline bromide ferrocene (3.38g, 9mmol) was added to the dry reaction flask, dissolved in 55mL ether, TMEDA (1.6mL, 10.8mmol) was added at -78°C, n-butyllithium ( 4.5mL, 2.4M, 10.8mmol) After stirring at this temperature for 2 hours, 3mL of P(OPh) 3 After being dissolved in 5 mL of ether, it was slowly added to the reaction, and the reaction was naturally raised to room temperature, and stirred overnight. The reaction solvent was ...
Embodiment 2
[0131]
[0132] Under nitrogen protection at room temperature, Pd 2 (dba) 3 (9.2mg, 0.01mmol) and ligand (9.6mg, 0.02mmol) were dissolved in 5mL DCE and stirred at room temperature for 30 minutes. Add allyl carbonate (96mg, 0.5mmol), dimethyl malonate (0.17mL, 1.5mmol), BSA (0.37mL, 1.5mmol) and NaOAc (1.0mg, 0.015mmol), react at room temperature, TLC tracking After the reaction is complete, dilute with DCM, quench by adding saturated ammonium chloride solution at 0°C, extract with DCM, combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain a crude product. Purified by column chromatography (petroleum ether: ethyl acetate = 10: 1) to obtain 88.2 mg of product, yield 71%, b / l = 10 / 90, 70% ee.
Embodiment 3 2
[0133] Example 3 Application of perfluoroalkyl P-N ligands of ferrocene skeleton in allylation reaction
[0134]
[0135] General experimental operation: under nitrogen protection at room temperature, Pd 2 (dba) 3 (9.2mg, 0.01mmol) and ligand (9.6mg, 0.02mmol) were dissolved in 5mL DCE and stirred at room temperature for 30 minutes. Add allyl carbonate (96mg, 0.5mmol), dimethyl malonate (0.17mL, 1.5mmol), BSA (0.37mL, 1.5mmol) and NaOAc (1.0mg, 0.015mmol), react at room temperature, TLC tracking After the reaction is complete, dilute with DCM, quench by adding saturated ammonium chloride solution at 0°C, extract with DCM, combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain a crude product. The ratio of the product was purified by column chromatography (petroleum ether: ethyl acetate = 10:1), and the ee value was determined by chiral HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com