Amphiphilic block copolymer and preparation method thereof, micelle drug delivery system formed by copolymer and anti-tumor drug
An amphiphilic block, drug-carrying system technology, applied in anti-tumor drugs, drug combinations, drug delivery, etc., can solve the problems of lack of safety of polymer excipients, poor drug stability, and differences in efficacy, and achieve good industrialization. Application prospects, the effect of increasing the force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 amphiphilic block copolymer
[0064] (1) Synthesis of benzoyl-terminated methoxypolyethylene glycol-polylactide block copolymer (mPEG 3400 -PLA 1800 -Bz)
[0065] Add 50g of mPEG (number average molecular weight 3400) into the polymerization bottle, heat to 100°C for 2h and vacuum dehydrate, then add 36mg of stannous octoate and 36g of D,L-lactide (D,L-LA), vacuum seal the reaction bottle, The above reactants were reacted at 125°C for 15 hours, then dissolved in dichloromethane, added a large amount of ether to fully precipitate the polymer, filtered, and vacuum-dried to obtain mPEG 3400 -PLA 1800 block copolymers.
[0066]Take 38g mPEG 3400 -PLA 1800 Add 190ml of ethyl acetate to dissolve, add 2.8ml of triethylamine, add 2.3ml of benzoyl chloride dropwise under stirring, heat the above solution to boiling after the dropwise addition, continue the reflux reaction for 6h, filter to remove insoluble matter, add a large amount of ether...
Embodiment 2
[0115] Example 2 Preparation of antineoplastic drug polymer micelles freeze-dried preparation
[0116] (1) mPEG 5000 -PCL 4000 -Ac / paclitaxel micelles and their lyophilized preparations
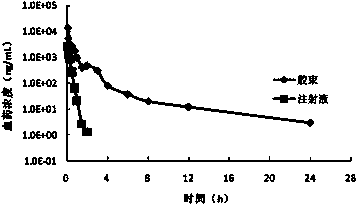
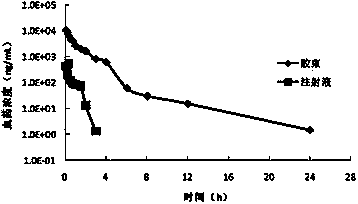
[0117] Get the mPEG prepared in 150mg embodiment 1 5000 -PCL 4000 -Ac and 30mg paclitaxel were dissolved in 2ml tetrahydrofuran, and 5ml ultrapure water was slowly added dropwise under stirring. After the dropwise addition, the solution was stirred at room temperature overnight to remove the organic solvent to obtain a clear paclitaxel micelle solution with obvious blue opalescence. 120 mg of mannitol was added, and the resulting solution was filtered through a 0.22 μm sterile membrane, and then freeze-dried to obtain paclitaxel micellar freeze-dried powder. According to LC-MS / MS analysis, the drug encapsulation rate is 96.5%, the drug loading is greater than 15.4%, and the particle size measurement results are as follows: Figure 9 As shown in , the average particle size is 28.7nm, and ...
Embodiment 3
[0131] Embodiment 3 stability test
[0132] (1) mPEG 5000 -PCL 4000 -Ac / paclitaxel micelles stability test
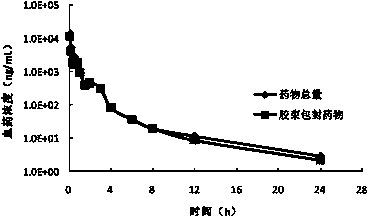
[0133] mPEG 5000 -PCL 4000 -Ac / paclitaxel micellar lyophilized powder reconstituted (concentration: 6mg / ml, calculated as paclitaxel) was stored in a constant temperature box at 25°C, taken out after a certain period of time, centrifuged at 10,000rpm for 10min, and then determined by high-performance liquid chromatography Drug content in the supernatant.
[0134] The content of the drug in the dissolved state changes with time as Figure 12 Shown: mPEG 5000 -PCL 4000 -The drug content in the Ac / paclitaxel micellar solution varies with time, and the drug in the dissolved state remains above 95% within 72 hours.
[0135] (2) mPEG 2000 -PLA 1800 -BP / docetaxel micellar stability test
[0136] mPEG 2000 -PLA 1800 - After reconstitution of BP / docetaxel micellar freeze-dried powder (concentration of 6mg / ml, calculated as docetaxel), store in a constant temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com