Alkaline pectate lyase (PelA) as well as encoding gene and application thereof
A technology of pectate lyase and coding gene, which is applied in the field of alkaline pectate lyase PelA and its coding gene and application, can solve the problems of increasing the complexity and cost of process conditions, and achieve good temperature and pH stability , high activity, safe and reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Cloning of Example 1 Gene
[0033]Extraction of RNA and preparation of template: The mycelium of the straw mushroom was inoculated on the rice straw medium for 8 days, and the mycelium was extracted with TRIZOL regent of invitrogen: the collected mycelium was ground into powder with liquid nitrogen, and an appropriate amount of powder was added to 1mL TRIZOL regent , after mixing evenly, let stand at room temperature for 5 minutes. Add chloroform, shake and mix well, and let stand at room temperature for 2-3 min. Centrifuge at 12000g for 15min. Transfer the supernatant to a clean centrifuge tube, add isopropanol, mix well and let stand at room temperature for 10 minutes, centrifuge at 12000g to discard the supernatant, wash the precipitate with 75% ethanol, and centrifuge at 7500g. Dry the RNA, pay attention to the fact that the RNA cannot be completely dried, dissolve the RNA with TE buffer, and incubate at 55-60°C for 10 minutes. The templates of 5' RACE and 3' RAC...
Embodiment 2
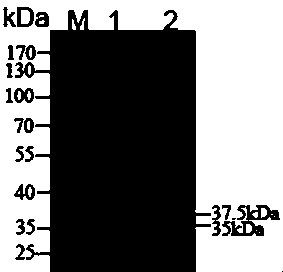
[0035] Example 2 Expression and purification of alkaline pectate lyase PelA in Pichia pastoris
[0036] Alkaline pectate lyase PelA was expressed using EasySelect Pichia Expression Kit (invitrogen). Two primers 5'-TGCCG were designed respectively GAATTC CGATTCCGATCGAACCCTCTGAGCA -3’ and 5’- GC TCTAGA TTAATGATGATGATGATGATGATGAAATGTCAAGGTCTGGCCG -3', using the PCR method to introduce two restriction enzyme sites EcoRI and XbaI at the N-terminal and C-terminal of xylanase respectively, after double-digestion, use the same double-digestion expression vector pPICZαB T4 DNA ligase ligation, the ligated recombinant plasmid pPICZαB--PelA was linearized by restriction endonuclease SacI and then electroporated into Pichia pastoris After KM71H, positive clones were screened using YPD plates containing 1 M sorbitol and 100 μg / mL Zeocin (Invitrogen) antibiotics. Insert the screened positive clones into a test tube containing 3mL YPD and 3μL antibiotic Zeocin at 28°C-30°C and culture...
Embodiment 3
[0038] Example 3 Activity Analysis of Alkaline Pectate Lyase PelA
[0039] Determination of pectate lyase activity: put the reaction system without enzyme (990μL reaction solution, containing 0.2% polygalacturonic acid (PGA), 100mM Glycine-NaOH buffer solution at pH 10.0) at 60 Preheat in a constant temperature water bath at ℃ for 5 minutes, add 0.1 mL of purified alkaline pectate lyase PelA (about 1 μg, prepared in Example 2) that has been properly diluted to the reaction solution, and react for 10 minutes, then add 500 mM hydrochloric acid Stop the reaction with 0.5mL of stop solution, centrifuge, take the supernatant, and measure the absorbance value A at 235 nm 235 . One enzyme activity unit: the amount of enzyme required to produce 1 μmol of unsaturated product per minute.
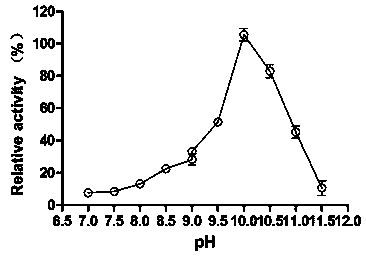
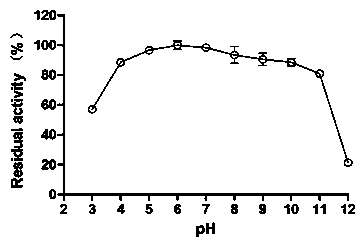
[0040] 1) Determination of the optimum pH and pH stability of alkaline pectate lyase PelA
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com