Synthesis method of RAFT chain transfer agent containing terminal hydroxyl
A technology of chain transfer agent and terminal hydroxyl group is applied in the field of organic synthesis of RAFT chain transfer agent, which can solve the problem that RAFT chain transfer agent cannot be directly applied, and achieve the effect of fast reaction rate and simple and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
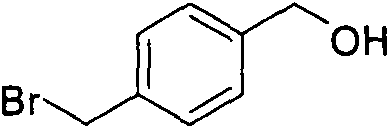
[0030] 4-bromomethylbenzyl alcohol can be purchased directly, or prepared by the following experimental method:
[0031] Dissolve 2.34 g of methyl p-bromomethylbenzoate in dry 15 mL of CH 2 Cl 2 , and cooled to 0 ° C, magnetic stirring, nitrogen protection. Slowly add 22 mL of DIBAL-H n-hexane solution dropwise with a dropping funnel. After the dropwise addition, continue stirring at 0°C for 10 minutes, and then react at room temperature for 24 hours. After the reaction, add 30mLCH to the reaction solution 2 Cl 2 , pour the mixture into the dropping funnel, and slowly drop it into a mixture of 30% hydrochloric acid and crushed ice, and stir it with a magnetic force. The organic phase was separated and the aqueous phase was extracted twice with 20 mL of ether. The mixed organic phase was washed 3 times with saturated sodium bicarbonate solution, then washed 3 times with saturated sodium chloride solution, and the finally obtained organic phase was washed with MgSO 4 Dryin...
Embodiment 2
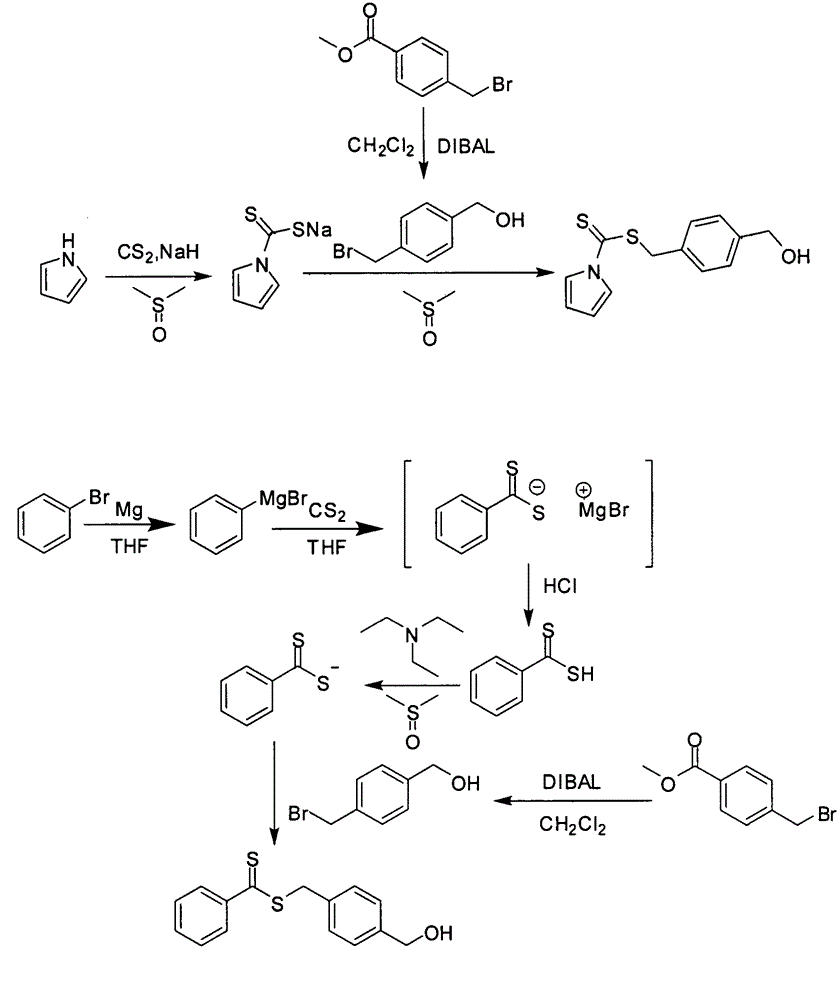
[0033] (0.48g, 20mmol) of sodium hydride was added to a 100mL round bottom flask filled with 20mL of dimethyl sulfoxide, and (1.4mL, 20mmol) of pyrrole was slowly added dropwise with a dropping funnel. After the addition, the brown reaction solution was magnetically stirred at room temperature for 30 minutes, and then carbon disulfide (1.2 mL, 20 mmol) was slowly added dropwise. The reaction solution continued to be magnetically stirred at room temperature for 30 minutes, then weighed 4 g of 4-bromomethylbenzyl alcohol, dissolved in a small amount of dimethyl sulfoxide, and slowly added dropwise with a dropping funnel. After reacting for 1 hour, 20 mL of water was added, followed by 20 mL of diethyl ether. The orange liquid layer was separated, the aqueous phase was extracted twice with 20 mL of diethyl ether, the combined organic phases were dried over anhydrous magnesium sulfate, filtered and the solvent was removed. The crude product was chromatographed with 10% ethanol an...
Embodiment 3
[0035] Dithiobenzoic acid can be purchased directly, prepared by other synthetic methods, or prepared by the following experimental method:
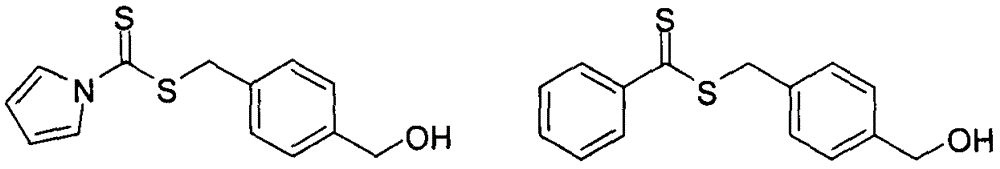
[0036] Take bromobenzene (62.8g, 0.40mol) and magnesium chips (10g, 0.42mol) and add them to a 500mL round-bottomed flask filled with 300mL of dry tetrahydrofuran, and heat to 40°C, and (30.4g, 0.41mol) of carbon disulfide Add dropwise slowly over 15 minutes, maintaining a reaction temperature of 40°C. After reacting for 1 hour, cool, add 30% hydrochloric acid to mix, then pour into a separatory funnel, extract with ether, the obtained ether solution is dried with anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain violet dithiobenzoic acid, the product structure through 1 H NMR with 13 C NMR identification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com