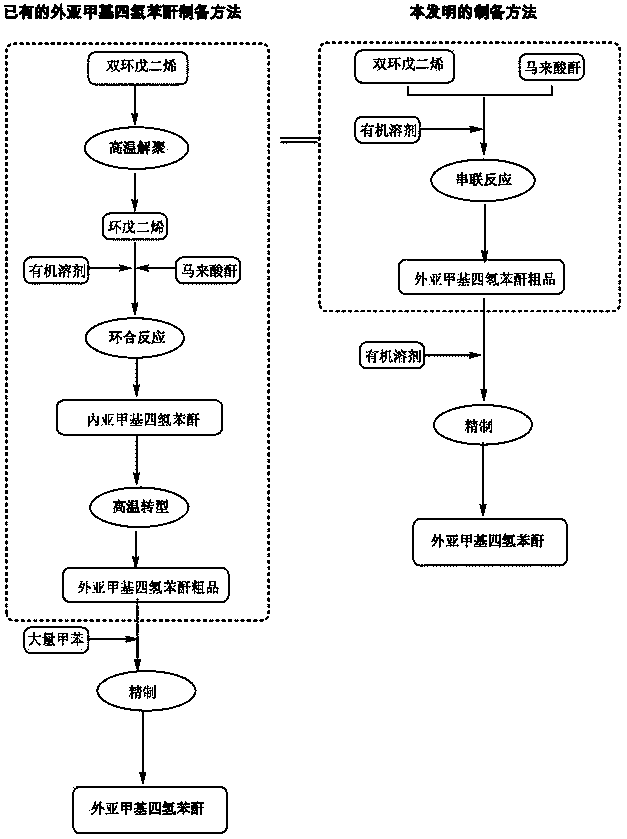
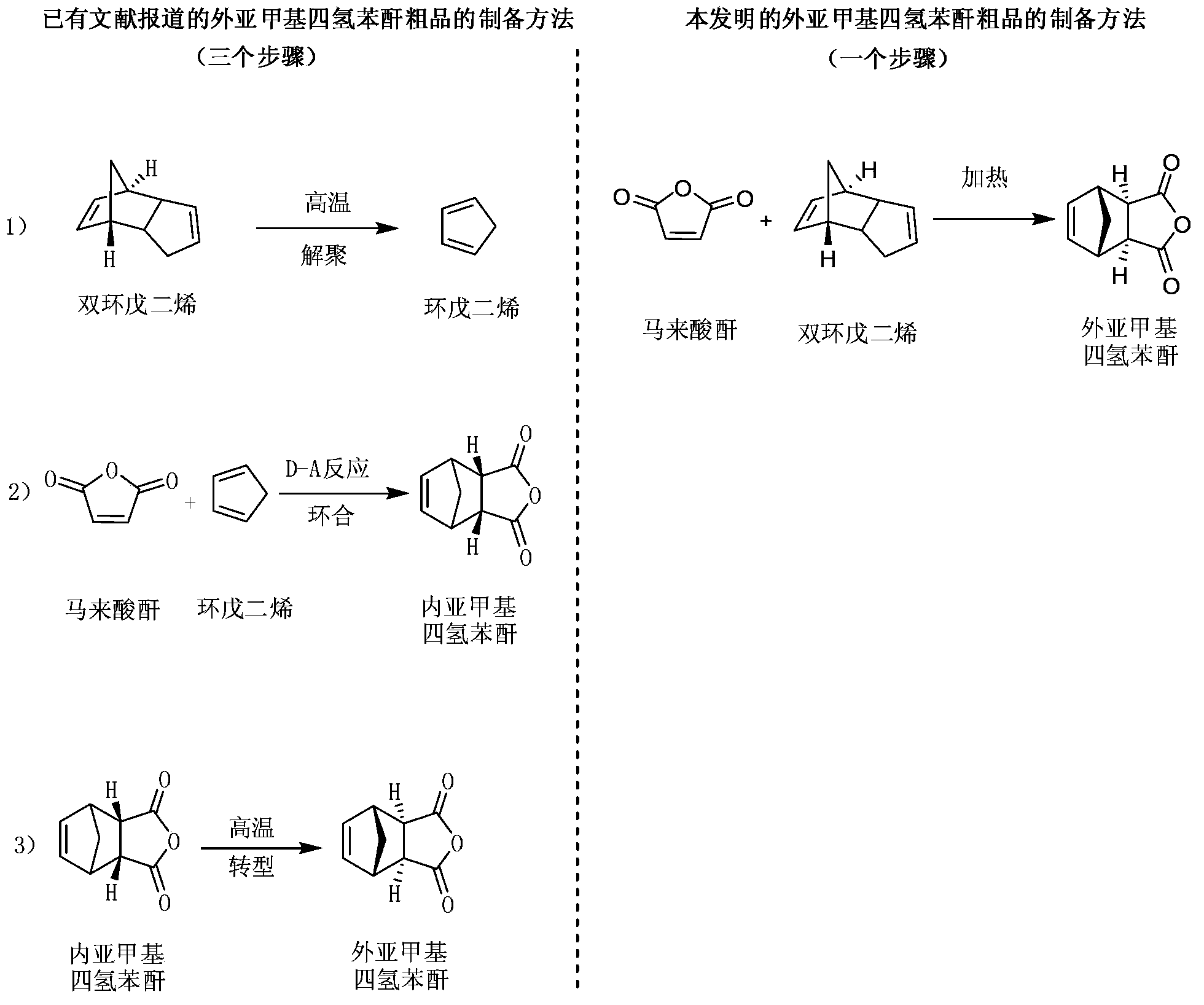
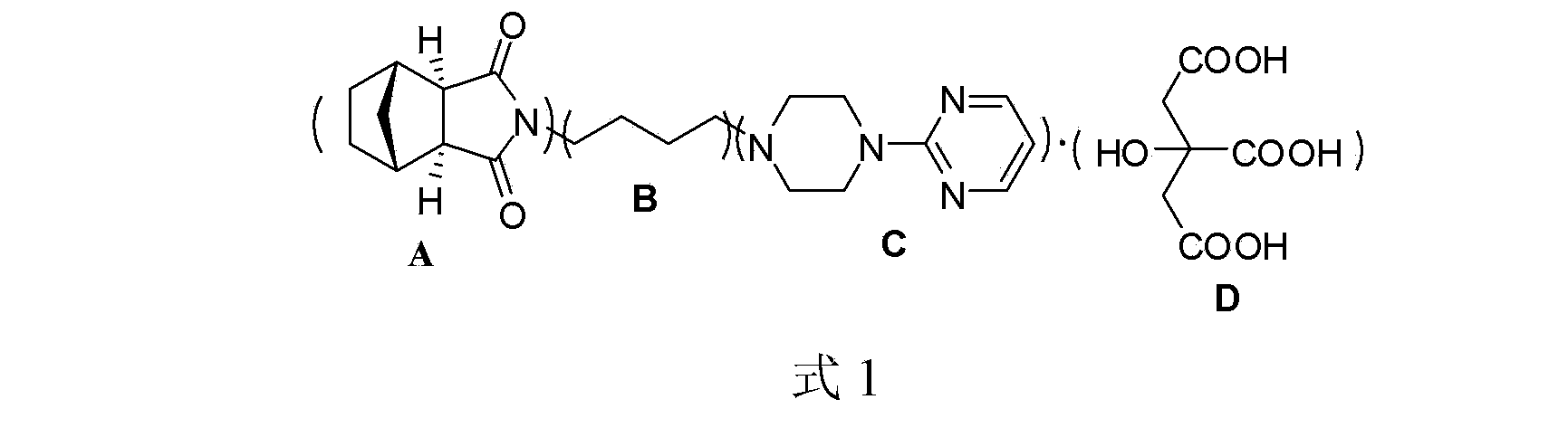
Preparation and refining method of exo-methylene tetrahydrophthalic anhydride and its use in preparation of tandospirone
A technology of exo-methylene tetrahydrophthalic anhydride and methylene tetrahydrophthalic anhydride, which is applied in the field of compound synthesis, can solve the problems of low refining yield, environmental threat, low transformation total yield, etc., achieves high crude product quality, and solves toxicity big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of exo-methylenetetrahydrophthalic anhydride
[0062] Weigh 98 g of maleic anhydride and 26 g of dicyclopentadiene, place them in the reaction kettle, add 100 mL of dichloromethane, seal, stir, and raise the temperature to 150°C. After the reaction is complete, the resulting reaction solution is cooled and crystallized , Separated and dried, 47.5g crude exo-methylene tetrahydrophthalic anhydride (yield 72.35%) is obtained.
Embodiment 2
[0065] Example 2 Preparation of exo-methylenetetrahydrophthalic anhydride
[0066] Weigh 98 g of maleic anhydride and 528 g of dicyclopentadiene, put them in the reaction kettle, add 2000 mL of dichloromethane, seal, stir, and raise the temperature to 220°C. After the reaction is complete, the resulting reaction solution is cooled and crystallized , Separation and drying, to obtain 144.7 g of crude exo-methylene tetrahydrophthalic anhydride (yield 88.26%).
[0067] The retention time of the main peak is consistent with the reference substance.
[0068] Chromatographic purity: 67.33%.
Embodiment 3
[0069] Example 3 Preparation of exo-methylenetetrahydrophthalic anhydride
[0070] Weigh 98 g of maleic anhydride and 793 g of dicyclopentadiene, put them in a reaction kettle, add 6000 mL of ethanol, seal, stir, and raise the temperature to 120°C. After the reaction is complete, the resulting reaction solution is cooled, crystallized, and separated After drying, 145.2g of crude exo-methylenetetrahydrophthalic anhydride is obtained (yield 88.51%).
[0071] The retention time of the main peak is consistent with the reference substance.
[0072] Chromatographic purity: 67.54%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com