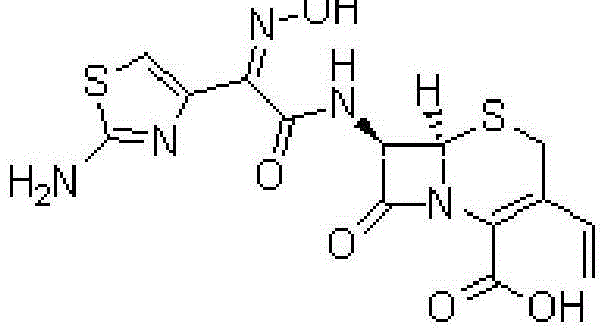
Cefdinir capsule and preparation method thereof
A cefdinir and capsule technology, applied in the field of pharmaceutical preparations and its preparation, can solve the problems of cumbersome process, easy hygroscopicity of cefdinir, and poor solubility of cefdinir
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Example 1: (the best formula obtained after screening)
[0044] Cefdinir 100 parts
[0045] Microcrystalline cellulose 80 parts
[0046] 6 parts of croscarmellose sodium
[0047] Sodium starch glycolate 7 parts
[0048] 6 parts of silicon dioxide
[0049] Sodium Lauryl Sulfate 6 parts
[0050] 5 parts crospovidone
[0051] 2 parts magnesium stearate
[0052] Process description:
[0053] Step 1, pulverize the former powder of cefdinir with a superfine pulverizer, adjust the frequency conversion of the air intake of the superfine pulverizer and the frequency conversion of the host speed to make it reach a dynamic balance, and collect the selected raw powder after the micropowder. Use MASTERSIZER2000 instrument to detect the particle size of cefdinir raw powder, make the particle size reach d(0.5) in the range of 10-25um.
[0054] Step 2, the cefdinir raw material is passed through an 80-mesh sieve for subsequent use. Other pharmaceutical excipients are sieved th...
example 2
[0060] Cefdinir 100 parts
[0061] Microcrystalline cellulose 50 parts
[0062] 13 parts of croscarmellose sodium
[0063] Carboxymethyl starch sodium 11 parts
[0064] 12 parts of silicon dioxide
[0065] Sodium Lauryl Sulfate 12 parts
[0066] 15 parts of crospovidone
[0067] 1 part magnesium stearate
[0068] Process description:
[0069] Step 1, pulverize the former powder of cefdinir with a superfine pulverizer, adjust the frequency conversion of the air intake of the superfine pulverizer and the frequency conversion of the host speed to make it reach a dynamic balance, and collect the selected raw powder after the micropowder. Use MASTERSIZER2000 instrument to detect the particle size of cefdinir raw powder, make the particle size reach d(0.5) in the range of 10-25um.
[0070] Step 2, the cefdinir raw material is passed through an 80-mesh sieve for subsequent use. Other pharmaceutical excipients are sieved through an 80 mesh sieve for later use.
[0071] Step 3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com