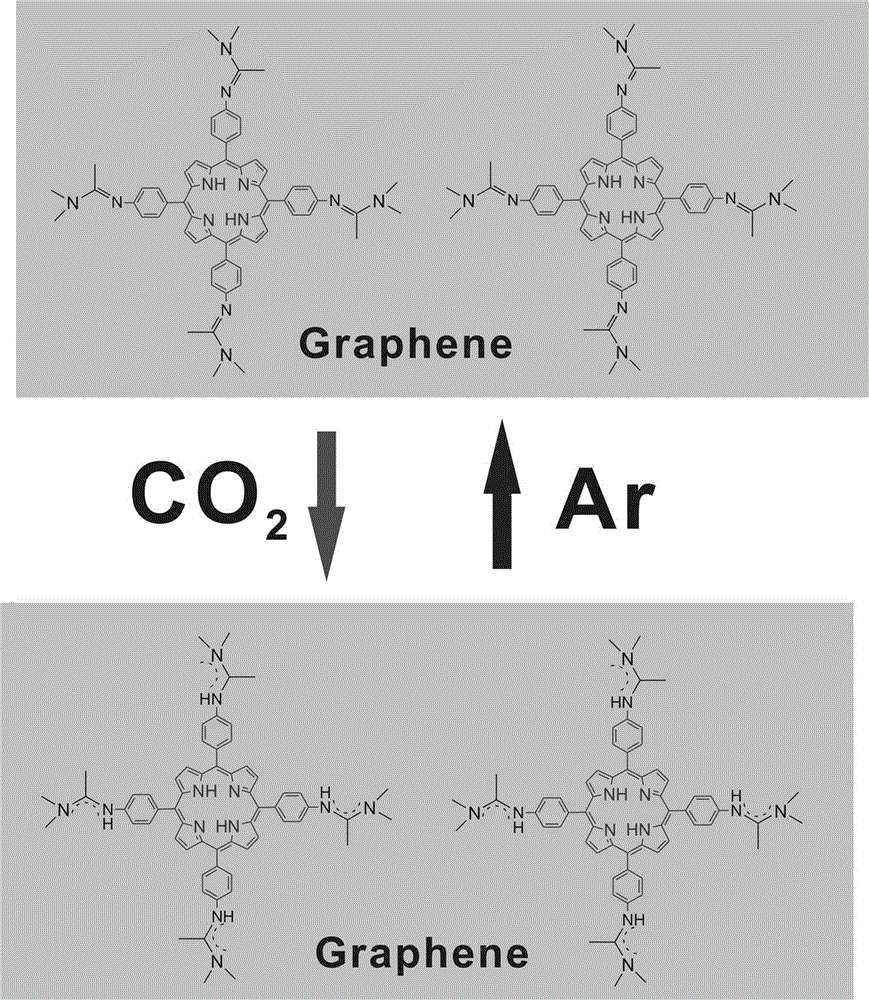Preparation method of carbon dioxide responded graphene nano hybrid material
A technology of nano-hybrid materials and carbon dioxide, which is applied in the field of smart materials and nano-materials, and achieves the effect of simple synthesis methods and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Dissolve 0.67 g of 5,10,15,20-tetrakis(4-aminophenyl)-21H,23H-porphyrin with 1.06 g of 1,1-dimethoxy-N,N-dimethylethylamine in Solvent N,N-dimethylformamide. Under the protection of argon, 40 o C for 24 hours. The solution was then cooled to room temperature, and the solvent N,N-dimethylformamide was removed under reduced pressure. Then add 15 ml of mixed solvent composed of chloroform and water, the volume ratio of chloroform and water is 1:1. Carbon dioxide was bubbled through the solution for 10 minutes. The aqueous phase is subsequently separated. To the aqueous phase was added 10 ml of solvent chloroform, followed by argon for 10 minutes. The resulting organic phase was separated. Add precipitant cyclohexane to the organic phase for precipitation, and the volume ratio of cyclohexane to organic phase is 5:1 to obtain 5,10,15,20-tetrakis(4-phenyl-N,N-dimethyl- N''-acetamidine bicarbonate)-21H,23H-porphyrin; 0.50 g of 5,10,15,20-tetrakis(4-phenyl-N,N-dimethyl-N...
Embodiment 2
[0019] Dissolve 0.67 g of 5,10,15,20-tetrakis(4-aminobenzene)-21H,23H-porphyrin with 1.02 g of 1,1-dimethoxy-N,N-dimethylethylamine in Solvent N,N-dimethylacetamide. Under the protection of argon, 50 o C for 18 hours. The solution was then cooled to room temperature, and the solvent N,N-dimethylacetamide was removed under reduced pressure. Then add 20 ml of mixed solvent consisting of dichloromethane and water, the volume ratio of dichloromethane and water is 1:1. Carbon dioxide was bubbled through the solution for 15 minutes. The aqueous phase is subsequently separated. 20 ml of solvent dichloromethane was added to the aqueous phase, and argon was passed through for 15 minutes. The resulting organic phase was separated. Add precipitant n-hexane to the organic phase for precipitation, the volume ratio of n-hexane to organic phase is 6:1, and obtain 5,10,15,20-tetrakis(4-phenyl-N,N-dimethyl-N' '-acetamidine bicarbonate)-21H,23H-porphyrin; Hydrogen salt)-21H, 23H-porphyr...
Embodiment 3
[0021] Dissolve 0.67 g of 5,10,15,20-tetrakis(4-aminobenzene)-21H,23H-porphyrin with 2.12 g of 1,1-dimethoxy-N,N-dimethylethylamine in solvent dimethyl sulfoxide. Under argon protection, 60 oC for 16 hours. The solution was then cooled to room temperature and the solvent dimethyl sulfoxide was removed under reduced pressure. Then add 30 ml of mixed solvent composed of dichloromethane and water, the volume ratio of dichloromethane and water is 1:1. Carbon dioxide was bubbled through the solution for 20 minutes. The aqueous phase is subsequently separated. To the aqueous phase was added 30 ml of solvent chloroform, followed by argon for 30 minutes. The resulting organic phase was separated. Add precipitant n-heptane to the organic phase for precipitation, the volume ratio of n-heptane to organic phase is 8:1, and obtain 5,10,15,20-tetrakis(4-phenyl-N,N-dimethyl-N ''-acetamidine bicarbonate)-21H,23H-porphyrin; 0.5 g of 5,10,15,20-tetrakis(4-phenyl-N,N-dimethyl-N''-acetamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com