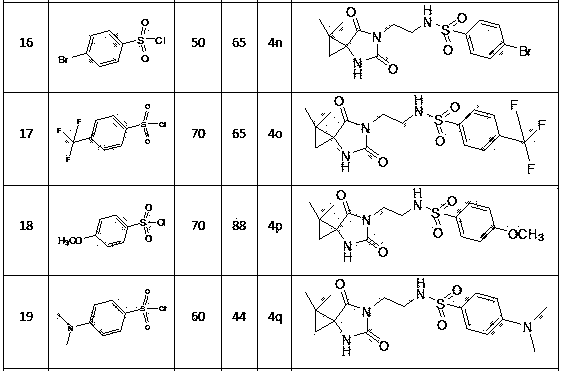
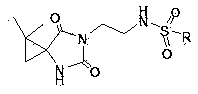
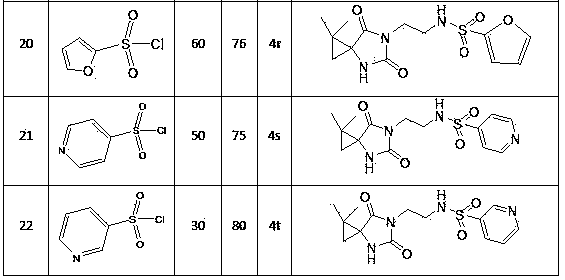
N-3-sulfonyl ethylamine substituted-5-cyclopropane spiro-hydantoin derivative as well as preparation method and application thereof
A technology of spirohydantoin and sulfonylethylamide, which can be used in drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, etc., and can solve problems such as cognitive dysfunction, severe allergic reactions, and adverse reactions. , to achieve the effect of high yield and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Synthesis of ethyl 1-isocyanate-2,2-dimethylcyclopropanecarboxylate (compound 2)
[0020] Dissolve ethyl 1-carboxy-2,2-dimethylcyclopropanecarboxylate (10 mmol) in anhydrous tetrahydrofuran (30 mL), cool to about -15°C in an ice-salt bath, and then add ethyl chloroformate sequentially (10mmol) and N-methylpyrrolidone (NMM), immediately produced a white precipitate. After the mixture was stirred at this temperature for 20 minutes, NaN3 (10 mmol) was added to the reaction solution, and stirring was continued for 2 hours. After the reaction is complete, add a small amount of water to dissolve the insoluble matter, extract with ethyl acetate, wash with saturated brine (2×10 mL), and dry overnight with anhydrous Na2SO4. After filtration, the solvent was evaporated under reduced pressure to obtain a pale yellow liquid. Dissolve it in toluene (30 mL), install a spherical condenser, oil seal, and heat under stirring until no gas is generated. The solvent is evaporated...
Embodiment 2
[0021] Example 2: Synthesis of 6-(2-aminoethyl)-1,1-dimethyl-4,6-diazaspiro[2.4]heptane-5,7-dione (compound 3)
[0022] At 5°C, the colorless thick liquid compound obtained in Example 1 was dissolved in anhydrous tetrahydrofuran, and the resulting solution was added dropwise to 100mml of ethylenediamine in 50ml of THF until the reaction was complete (the end of the reaction was monitored by TLC). Remove the solvent by pressure distillation, use dichloromethane / methanol as the eluent, and immediately column chromatography to obtain compound 3, namely 6-(2-aminoethyl)-1,1-dimethyl-4,6-diazepine Spiro[2.4]heptane-5,7-dione.
Embodiment 3
[0023] Example 3: N-(2-(1,1-Dimethyl-5,7-dioxo-4,6-diazaspiro[2.4]hept-6-yl)ethyl)methanesulfonamide ( Synthesis of compound 4a)
[0024] Dissolve 6-(2-aminoethyl)-1,1-dimethyl-4,6-diazaspiro[2.4]heptane-5,7-dione (10 mmol) in dry dichloromethane (30 mL), cool to about -5°C in an ice-salt bath, then add triethylamine (30mmol), at this temperature, add dropwise methylene chloride solution dissolved in methanesulfonyl chloride. Continue to stir until the reaction is complete (TLC detection). After the reaction is complete, add a small amount of water to dissolve the insoluble matter, extract with ethyl acetate, wash with saturated brine (2×10 mL), and dry overnight with anhydrous Na2SO4. After filtration, the solvent was evaporated under reduced pressure to obtain a pale yellow liquid. Transfer the crude product to a silica gel column, use petroleum ether / ethyl acetate as eluent, collect the components at Rf = 0.3, and evaporate the solvent under reduced pressure to obtain a whi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com