Phenylethanoid glycoside compound extracted from caryopteris clandonensis as well as preparation method and medical application thereof
A technology of phenylethanol glycosides and compounds, which is applied in the field of medicine, can solve the problems of the extraction and utilization of the active ingredients of eucalyptus aureus, which have not been reported, and achieve the enhancement of microglial cells to phagocytose myelin fragments, the preparation method is simple, and the pollution is less Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 phenylethanol glycosides
[0049] Take 12.0 kg of dried Herbs of Herba chrysalis, crush them, and extract them by cold soaking with 95% ethanol at room temperature (60 L, 1 week each time). The solvent was recovered under reduced pressure to obtain 1257 g of extract. Dissolve the medicinal extract with hot water, extract with n-butanol (2L each time), 438g of n-butanol, through MCI column chromatography, water, 10% methanol, 30% methanol, 50% methanol, 70% methanol , 95% methanol elution, TLC detection, combined the same components to obtain 6 components (Fr.1-Fr.6): Fr.1 (122g), Fr.2 (31g), Fr.3 (164g), Fr.4 (30g), Fr.5 (20g), Fr.6 (16g). The obtained camellia seed total saponins were eluted by Sephadex LH-20 with different proportions of methanol water system, reversed phase preparation liquid phase for further separation and purification, respectively eluted with different proportions of methanol water or acetonitrile water, detected...
Embodiment 2
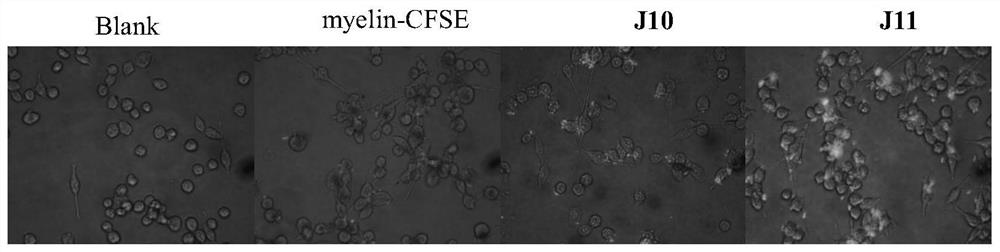
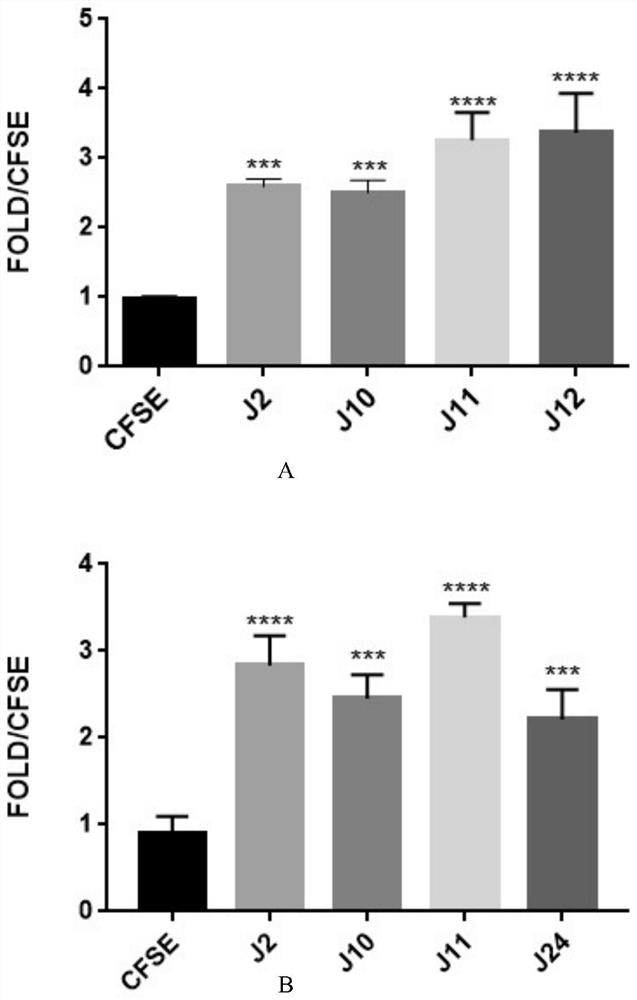
[0050] Example 2 Effect of monomer phenylethanol glycosides on myelin phagocytosis by microglia and astrocytes
[0051] 1. Method
[0052] (1) Cell culture
[0053] After recovery, microglia and astrocytes were cultured in DMEM containing 10% FBS, 1% p / s at 37°C, 5% CO 2cultured in a cell culture incubator. When the cells reach 90% confluence, wash once with PBS, add 1ml 0.25% trypsin to blow off, add culture medium after digestion is complete, transfer the digested cells into a centrifuge tube, centrifuge at 800rpm, 3min, remove the supernatant , add 1ml medium to resuspend the cells; according to the cell density, subculture into 1 or more culture dishes, and keep the mixed cells at 37°C CO 2 cultured in an incubator.
[0054] (2) Administration method and dosage
[0055] Take logarithmic growth phase glial cells, digest and inoculate in 96-well plate, 100 μl / well, 1×10 4 Each well, placed in the incubator for 12 hours after adherence, set up the blank group, the myeli...
Embodiment 3
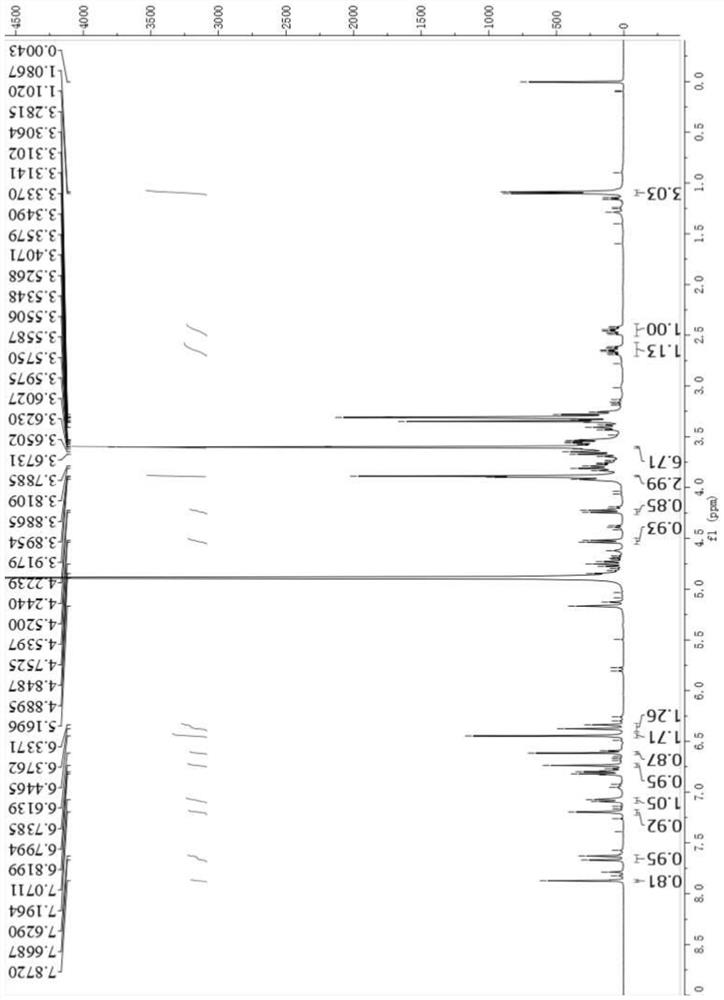
[0063] Example 3 Confirmation of the structure of the monomer phenylethanol glycoside J1
[0064] J1 is yellow-brown amorphous powder, easily soluble in methanol, (c 0.104, MeOH). By high resolution mass spectrometry HRESIMS m / z 997.2949[M-H] - (calcd.for 997.2954) and 1 H-NMR, 13 C-NMR spectral data can determine its molecular formula as C 46 h 54 o 23 , with an unsaturation of 20. 1 In the H-NMR spectrum (Table 1), it can be seen that there is a group of ABX coupling system aromatic hydrogen proton signal δ H 7.20(H,brs H-2'), δ H 6.81(1H,d,J=8.2Hz H-5') and δ H 7.08 (1H, d, J=8.2Hz H-6'), suggesting that J1 contains a 1,3,4-trisubstituted benzene ring; δ H 6.44(2H,s H-2”, H-6”) is the signal of two symmetrical aromatic hydrogen protons on the benzene ring, showing a 1,3,4,5-tetrasubstituted benzene ring; δ H 6.74 (1H, s H-2) and 6.61 (1H, sH-5) two singlet aromatic hydrogen signals suggest the presence of 1,3,4,6-tetrasubstituted benzene rings. Simultaneous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com