Method for detecting ferric ions through fluorescence
A technology of ferric iron and ions, applied in fluorescence/phosphorescence, biological testing, material inspection products, etc., can solve the problems of reduced fluorescence intensity and no fluorescence response, and achieve the effect of reduced fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 coumarin derivative 7-diethylaminocoumarin-4-formaldehyde
[0025] 7-Hydroxycoumarin-4-carbaldehyde was synthesized according to the literature, and its synthetic route:
[0026]
[0027] In xylene solution, add SeO 2 and 7-diethylamino-4-methylcoumarin (2.06 g, 8.9 mmol). Stirring was started, and the reaction mixture was heated to reflux for 3h. After the brown mixture cooled to room temperature, another portion of SeO was added 2 , the system continued to reflux for 3h. The dark brown residual oil was dissolved with acetone and the solid was filtered off. Concentration and purification by column chromatography in a hexane:acetone (4:1) solution system gave a dark yellow solid. Separation and purification yielded 620 mg of 7-diethylaminocoumarin-4-carbaldehyde with a yield of 28%.
[0028] 7-Diethylaminocoumarin-4-carbaldehyde 1 H NMR, 13 C NMR characterization, the results are as follows:
[0029] 1 H NMR (300MHz, CDCl 3 ...
Embodiment 2
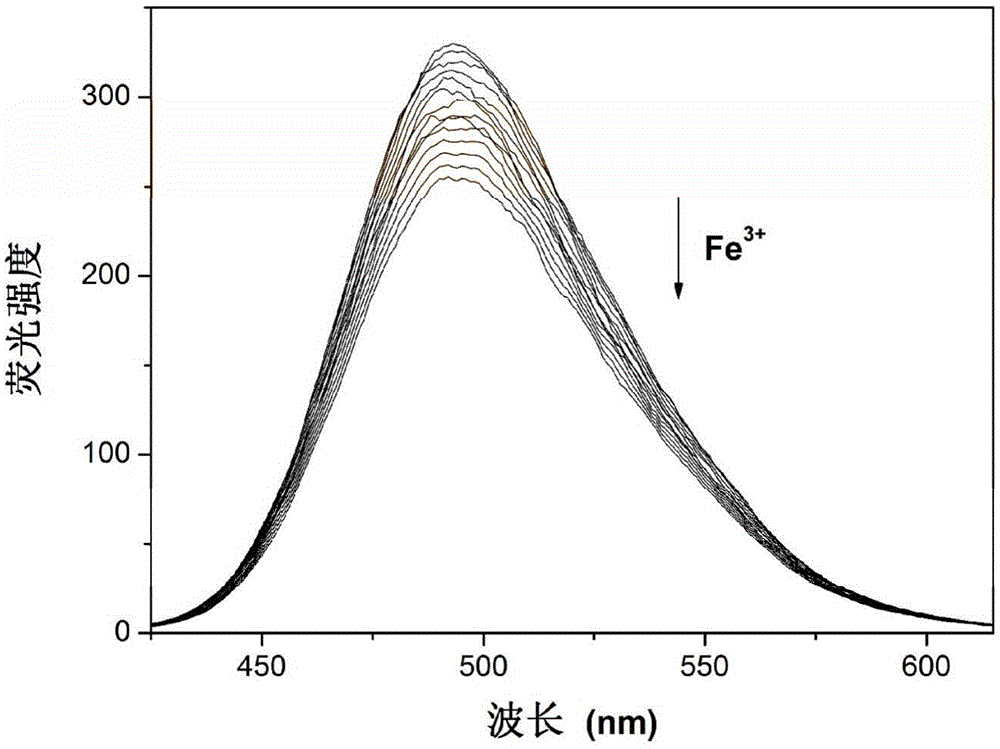
[0031] Example 2 Fe 3+ Fluorescence titration of 7-diethylaminocoumarin-4-carbaldehyde compound
[0032] In a fluorescent cuvette, add 1 μL of Fe every 10 s to 2 mL of HEPES buffer (pH=7.0) containing 7-diethylaminocoumarin-4-formaldehyde with a pipette gun 3+ For fluorescence titration experiments, a total of 18 μL was added. Detected on a fluorescence spectrophotometer, with the addition of the sample to be tested, the fluorescence intensity at 494nm is gradually weakened. Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 10.0nm and 10.0nm respectively, and in aqueous solution, the maximum excitation wavelength of the fluorescent probe solution is: λ ex 396nm and maximum fluorescence emission wavelength: λ em 494nm.
Embodiment 37- 2
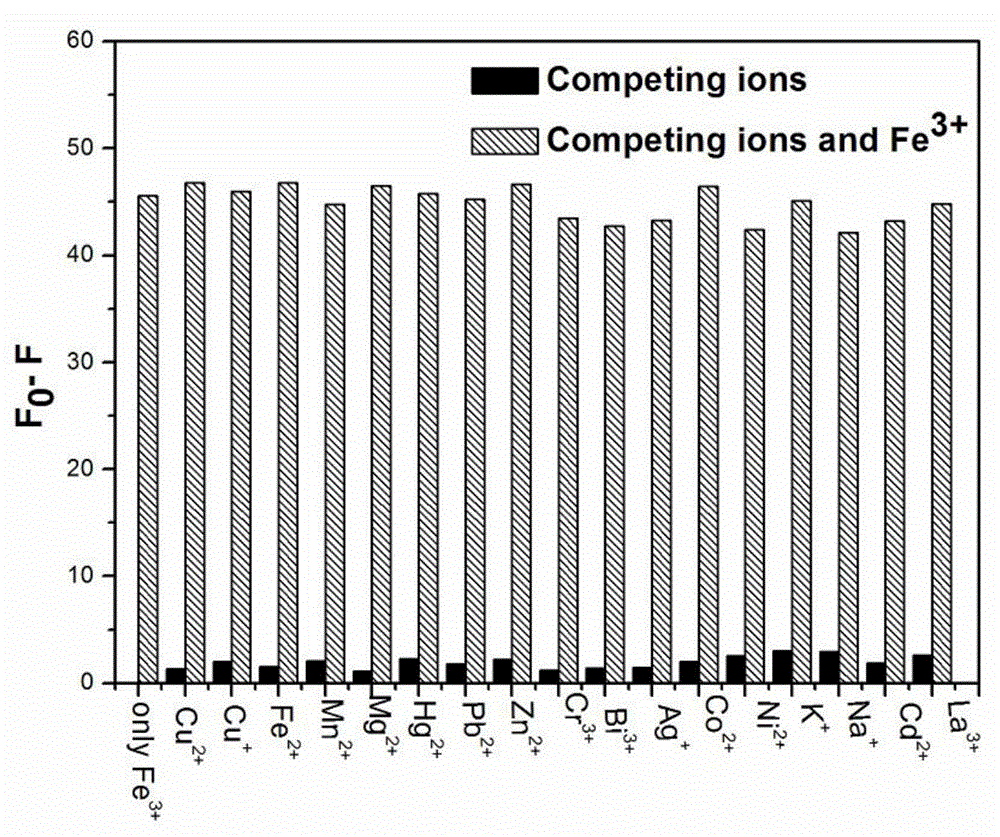
[0033] Embodiment 37-Diethylaminocoumarin-4-formaldehyde compound to the fluorescence histogram of various metal cations
[0034] Add 2 mL of HEPES buffer solution with pH=7.0 and 21 μL of fluorescent stock solution of 7-diethylaminocoumarin-4-formaldehyde in the cuvette, and then add other metal ions (Cu 2+ 、Cu + , Fe 2+ , Mn 2+ , Mg 2+ , Hg 2+ , Pb 2+ , Zn 2+ 、Cr 3+ 、 Bi 3+ 、Ag + 、Co 2+ 、Ni 2+ 、K + 、Na + 、Cd 2+ , La 3+ ), so that the final concentration is 1.8mM, then add Fe 3+ , so that the final concentration is 90 μM, measure the fluorescence spectrum respectively, and draw the histogram of the 494nm fluorescence intensity corresponding to different metal cations, see figure 2 . It has been proved by experiments that other metal cations do not interfere with the reaction of the system on Fe 3+ detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com