Lithium ion battery positive electrode material composed of cerium oxide and carbon co-coated lithium vanadium phosphate and preparation method thereof
A carbon-coated technology for lithium vanadium phosphate and lithium ion batteries, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as poor high-rate charge and discharge performance, and achieve the effects of reducing corrosion, simple process, and fine particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
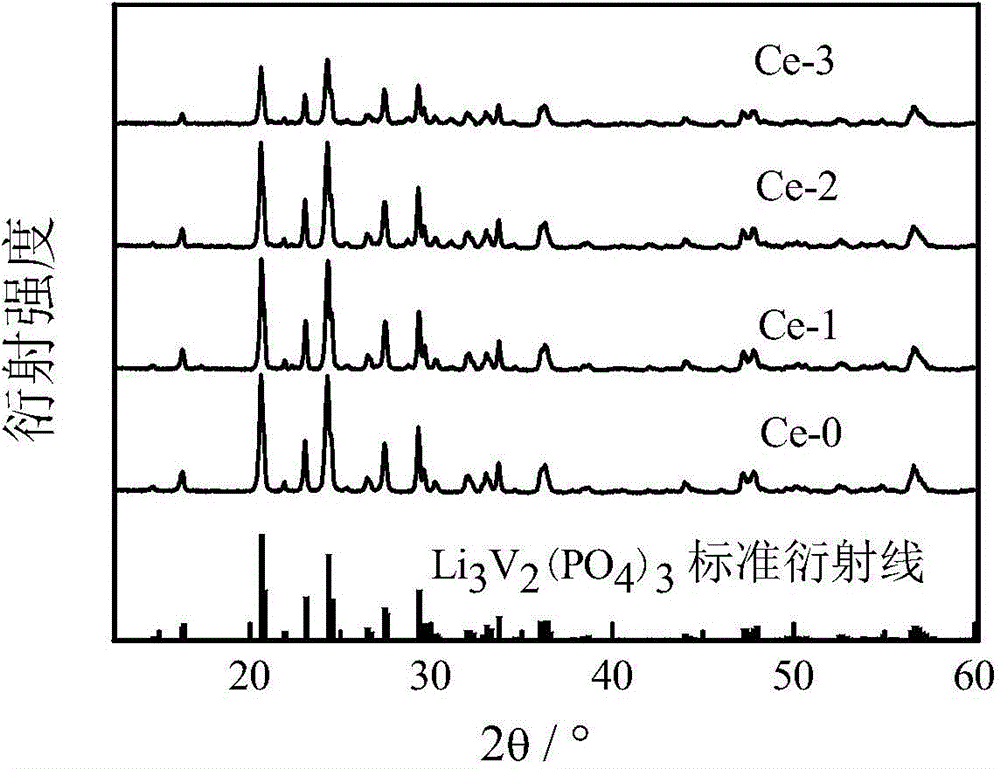
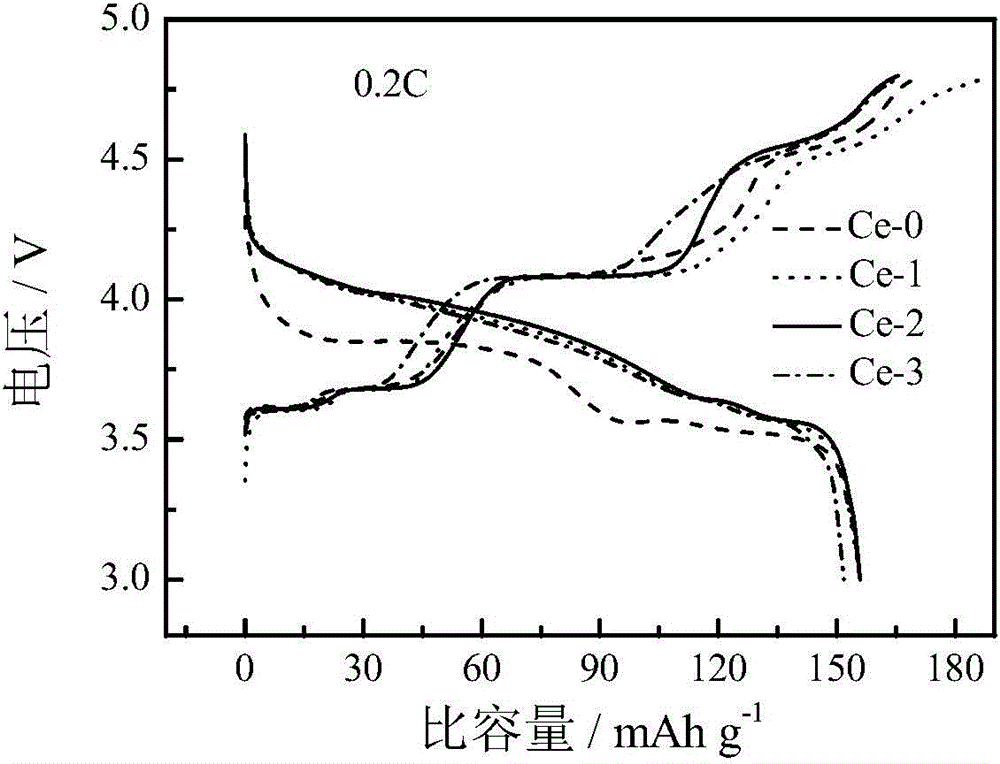
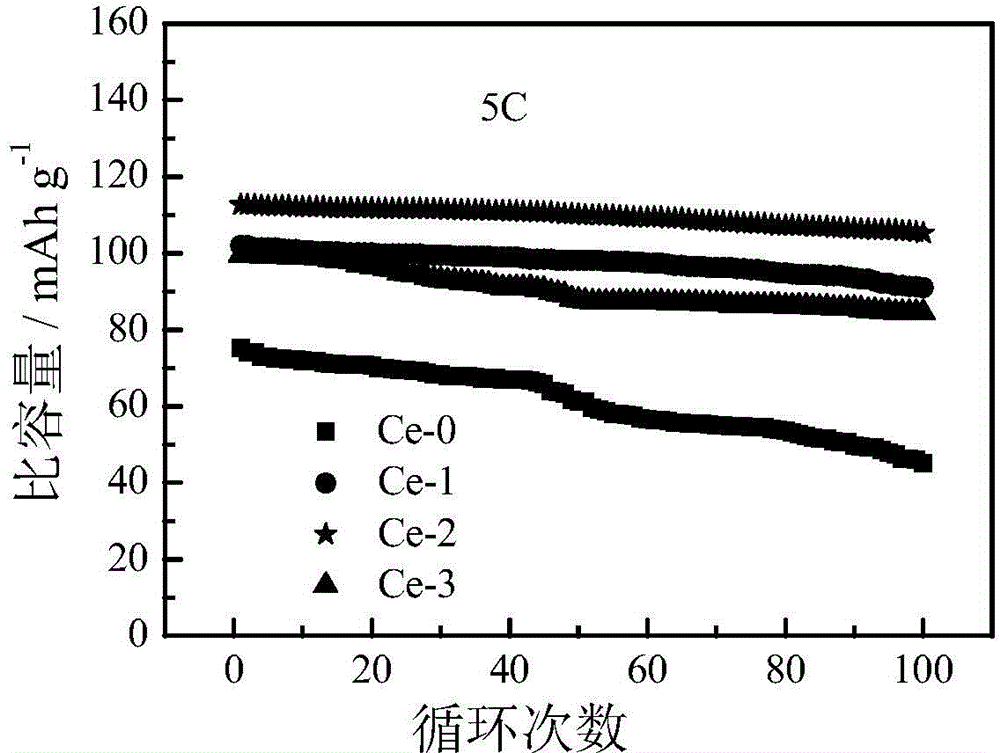
Image
Examples
Embodiment 1
[0037] Dissolve 4.145g of citric acid in 20mL of deionized water, pour it into a beaker containing 1.803g of vanadium pentoxide, and stir on a magnetic heating stirrer at a constant temperature of 60°C for 30 minutes to form a dark blue solution. Dissolve 3.421 g of ammonium dihydrogen phosphate and 1.121 g of lithium carbonate in 20 mL of deionized water respectively, add them to the above dark blue solution, and stir on a magnetic heating stirrer at a constant temperature of 60°C for 30 minutes. The pH value was adjusted to 4 with acetic acid, and the mixed liquid was stirred at a constant temperature of 60° C. for 1 hour on a magnetic heating stirrer. Raise the temperature to 80°C, continue stirring to evaporate the solvent to form a dark blue wet gel, and put it in an oven at 80°C for 1 day to form a dry gel. The xerogel was ground in a mortar for 30 minutes to form a blue-green powder. Then the blue-green powder is kept at 350° C. for 4 hours under a hydrogen-nitrogen mi...
Embodiment 2
[0041]Dissolve 4.145g of citric acid in 20mL of deionized water, pour it into a beaker containing 1.803g of vanadium pentoxide, and stir on a magnetic heating stirrer at a constant temperature of 60°C for 30 minutes to form a dark blue solution. Dissolve 3.421 g of ammonium dihydrogen phosphate and 1.121 g of lithium carbonate in 20 mL of deionized water respectively, add them to the above dark blue solution, and stir on a magnetic heating stirrer at a constant temperature of 60°C for 30 minutes. The pH value was adjusted to 4 with acetic acid, and the mixed liquid was stirred at a constant temperature of 60° C. for 1 hour on a magnetic heating stirrer. Raise the temperature to 80°C, continue stirring to evaporate the solvent to form a dark blue wet gel, and put it in an oven at 80°C for 1 day to form a dry gel. The xerogel was ground in a mortar for 30 minutes to form a blue-green powder. Then the blue-green powder is kept at 350° C. for 4 hours under a hydrogen-nitrogen mix...
Embodiment 3
[0045] Dissolve 4.145g of citric acid in 20mL of deionized water, pour it into a beaker containing 1.803g of vanadium pentoxide, and stir on a magnetic heating stirrer at a constant temperature of 60°C for 30 minutes to form a dark blue solution. Dissolve 3.421 g of ammonium dihydrogen phosphate and 1.121 g of lithium carbonate in 20 mL of deionized water respectively, add them to the above dark blue solution, and stir on a magnetic heating stirrer at a constant temperature of 60°C for 30 minutes. The pH value was adjusted to 4 with acetic acid, and the mixed liquid was stirred at a constant temperature of 60° C. for 1 hour on a magnetic heating stirrer. Raise the temperature to 80°C, continue stirring to evaporate the solvent to form a dark blue wet gel, and put it in an oven at 80°C for 1 day to form a dry gel. The xerogel was ground in a mortar for 30 minutes to form a blue-green powder. Then the blue-green powder is kept at 350° C. for 4 hours under a hydrogen-nitrogen mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com