Method for synthesizing dibornyl oxalate by using borneol
A diborneol oxalate and borneol technology, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of difficulty in synthesizing borneol, lack of diborneol oxalate, etc., and achieve easy separation and purification, low operating cost, easy to obtain effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
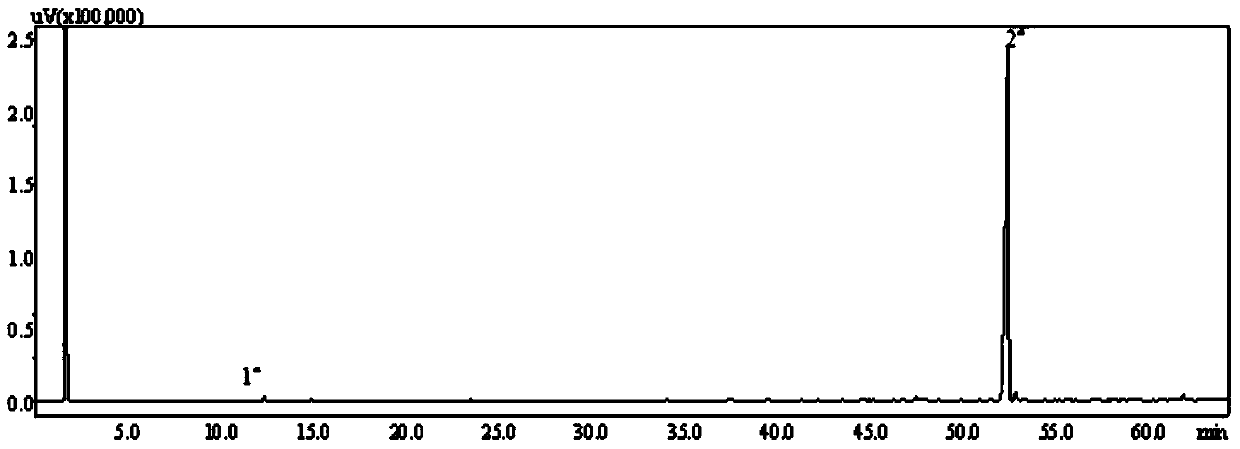
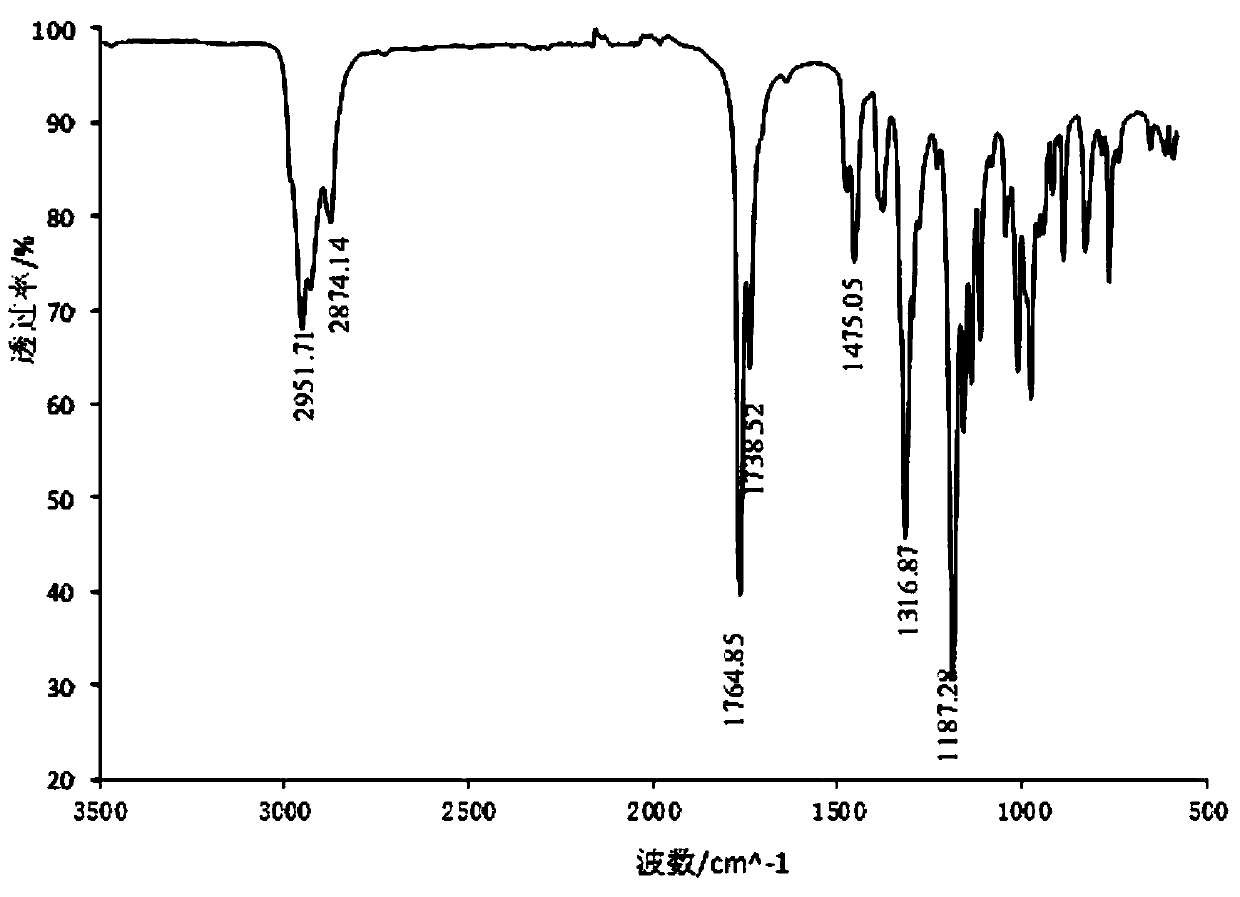
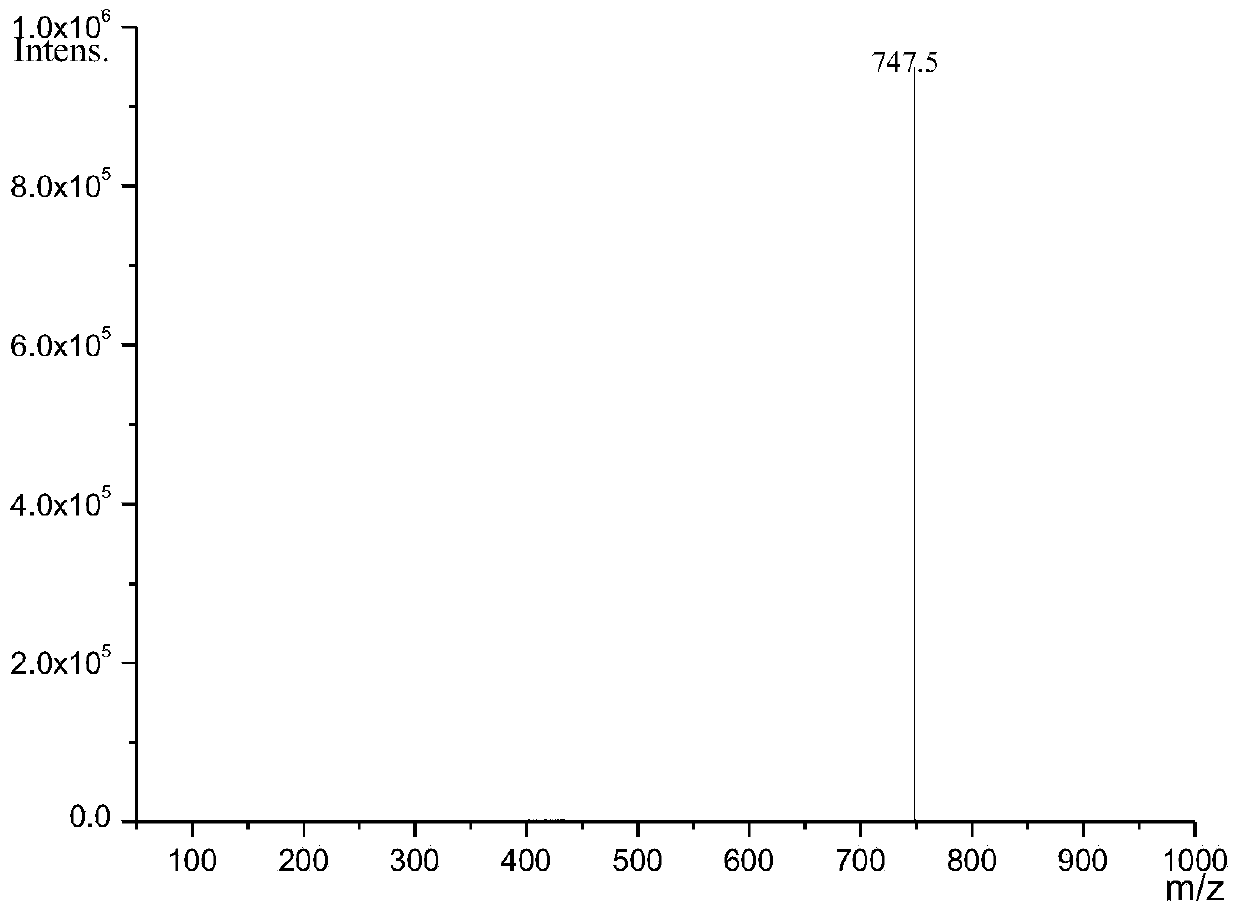
Image
Examples
Embodiment 1
[0044] With 7.5g (0.049mol) borneol (natural borneol, commercially available item, through recrystallization purification, ) was dissolved in 134g of n-hexane, reacted with 21.0g (0.23mol, the ratio of the amount of borneol to borneol was 4.8) anhydrous oxalic acid under the action of 3.1g boron anhydride catalyst for 25h at 55°C, and filtered to remove unreacted anhydrous oxalic acid Water oxalic acid and catalyst, and washed with water below 30°C for 5-6 times, the upper liquid was removed by rotary evaporation to remove most of the solvent, and then the unreacted raw material borneol was removed by steam distillation to obtain the crude product of dibornyl oxalate , and then use any one of acetone, ethanol, ethyl acetate, n-hexane, cyclohexane, etc. to obtain 1.5 g of dibornyl oxalate (0.0041 mol, the yield of the substance is 16.8%) through repeated recrystallization.
Embodiment 2
[0046] Dissolve 19.5g (0.13mol) borneol in 268.2g n-hexane, and mix with 55.5g (0.62mol, the ratio of borneol to 4.7) anhydrous oxalic acid under the action of 7.3g boron anhydride catalyst at 54-56 The reaction was carried out at °C for 115 h, and other conditions and operations were the same as those in Example 1 to obtain 8.2 g (0.0226 mol, yield of substance: 34.8%) of dibornyl oxalate.
Embodiment 3
[0048] 10.0g (0.065mol) borneol was dissolved in 91g cyclohexane and 69g light petroleum ether, and 8.6g (0.096mol, the ratio of the substance amount to borneol was 1.5) anhydrous under the action of 1.5g boric anhydride catalyst Oxalic acid was reacted at 60° C. for 24 h, and other conditions and operations were the same as in Example 1 to obtain 1.3 g of diborneyl oxalate (0.0036 mol, the yield of the substance was 11.0%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com