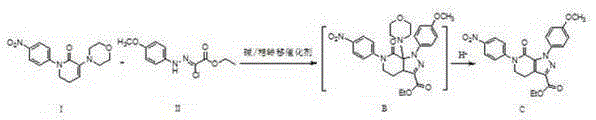
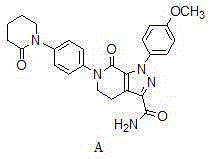
A kind of preparation method of Apixaban
A technology for apixaban and a compound, which is applied in the field of preparation of apixaban, can solve the problems of expensive chemical dehydration reagents, high moisture content requirements, unsuitable for industrialized production and the like, and achieves low production costs and low equipment requirements. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
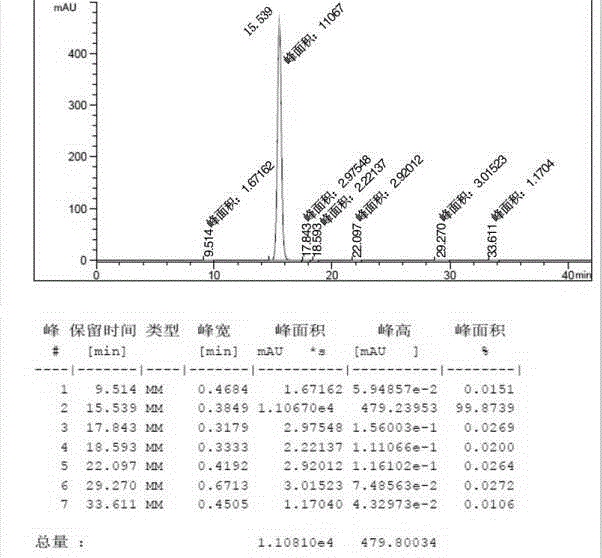
[0072] Embodiment 1: The preparation method of this apixaban adopts the following specific process steps.
[0073] (1) Synthesis of compound C:
[0074]
[0075] 50 mL of dichloromethane was added to the reaction flask, and intermediate I (5.00 g, 0.0165 mol), intermediate II (5.08 g, 0.0198 mol), sodium carbonate (5.25 g, 0.0495 mol) and tetrabutyl were sequentially added under stirring. Ammonium bromide (1.06 g, 0.0033 mol) was stirred at room temperature for 10 h after the reaction was confirmed by TLC to complete the reaction. Dilute hydrochloric acid was slowly added dropwise to the reaction flask until the pH of the system was 2-3, and the reaction was continued at room temperature for 2 h after TLC confirmed that the reaction was complete. The reaction was stopped, 50 mL of water was added and stirred, the organic layer was separated and washed with water (2×50 mL), and the organic layer was dried with anhydrous sodium sulfate. Suction filtration, the filtrate was ...
Embodiment 2
[0091] Embodiment 2: The preparation method of this apixaban adopts the following specific process steps.
[0092] (1) Synthesis of compound C:
[0093] 50 mL of dichloromethane was added to the reaction flask, and intermediate I (5.00 g, 0.0165 mol), intermediate II (4.67 g, 0.0182 mol), potassium carbonate (6.83 g, 0.0495 mol) and tetrabutyl were sequentially added under stirring. Ammonium bromide (1.06 g, 0.0033 mol) was stirred at a temperature of 20 °C for 10 h, and TLC confirmed that the reaction was complete. Cool in an ice-water bath, slowly add dilute hydrochloric acid dropwise to the reaction flask at 0°C until the pH of the system is about 2-3, and after 3 hours of temperature-controlled reaction, TLC confirms that the reaction is complete. The reaction was stopped, 50 mL of water was added and stirred, the organic layer was separated and washed with water (2×50 mL), and the organic layer was dried with anhydrous sodium sulfate. Suction filtration, the filtrate wa...
Embodiment 3
[0104] Embodiment 3: The preparation method of this apixaban adopts the following specific process steps.
[0105] (1) Synthesis of compound C:
[0106] 50 mL of ethyl acetate was added to the reaction flask, and intermediate I (5.00 g, 0.0165 mol), intermediate II (4.66 g, 0.0182 mol), triethylamine (4.33 g, 0.0413 ) and tetrabutyl were added in sequence under stirring. Ammonium bromide (0.80 g, 0.0025 mol) was heated to 70 °C and reacted for 5 h after TLC confirmed that the reaction was complete. After dropping to room temperature, dilute sulfuric acid was slowly added dropwise to the reaction flask until the pH of the system was about 2 to 3, and the reaction was continued at 70° C. for 0.5 h to confirm that the reaction was complete. The reaction was stopped, 50 mL of water was added and stirred, the organic layer was separated and washed with water (2×50 mL), and the organic layer was dried with anhydrous sodium sulfate. Suction filtration, and the filtrate was evaporat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com