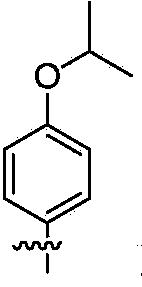
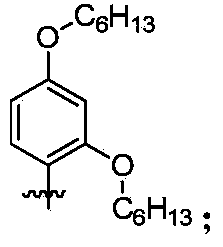
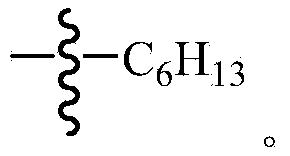Organic dye sensitizer, preparation method and application in photoelectric conversion
A technology of organic dyes and sensitizers, used in organic dyes, organic chemistry, tin organic compounds, etc., can solve problems such as poor light, thermal and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0443]
[0444]1) Synthesis of intermediate A1: N-bromosuccinimide (1.60 g, 9 mmol) was added to 3-formylthiophene (1.0 g, 9 mmol) in 80 mL of N,N-dimethylformamide solution, react at room temperature for 2 days, then add 100 mL of water, and extract with ethyl acetate (100 mL, 3 times), the organic layer is concentrated, and the silica gel column (petroleum ether: acetic acid Ethyl ester=50:1) to obtain 1.9 g of compound A1 with a yield of 78.5%. 1 H-NMR (400MHz, DMSO-d 6 ) δ: 7.476(s, 1H), 9.722(s, 1H). ESI-MS[M+H] + :267.8.
[0445] 2) Synthesis of intermediate B1: Add compound A1 (538 mg, 2 mmol), thiophene-2-boronic acid (256 mg, 2 mmol), tetrakistriphenylphosphine palladium (232 mg, 0.2 mmol) into a 100 ml flask mol) and tetrahydrofuran: water (5:1) 24 ml. Under nitrogen protection, the mixture was reacted at 70°C for 8 hours, cooled to room temperature, concentrated to remove the solvent, and silica gel column chromatography (petroleum ether: ethyl acetate = 50:...
Embodiment 2
[0452]
[0453]
[0454] 1) Synthesis of intermediate I: Add 1,1,7,7-tetramethyljulonidine (91.6 mg, 0.4 mmol), N-bromosuccinimide (71.2 g, 0.4 mmol) and 12 ml of dichloromethane. Under nitrogen protection, the mixture was reacted at room temperature for 16 hours, 12 ml of water was added, extracted with dichloromethane (15 ml, 3 times) and concentrated to remove the solvent to obtain 105 mg of compound I, LC-MS purity: 100%, yield 85.1 %. 1 H-NMR (400MHz, DMSO-d 6 )δ: 1.254(s, 12H), 1.733(t, 4H), 3.121(t, 4H), 7.083(s, 2H), ESI-MS (M+H + ): 310.0.
[0455] 2) Synthesis of Intermediate J: Compound I (92.4 mg, 0.3 mmol), double pinacol borate (152 mg, 0.6 mmol), potassium acetate (88.3 mg, 0.9 mmol) were added to a 50 ml flask ), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (Pd(dppf)Cl 2 ) (24 mg, 0.03 mmol) and 20 ml of dioxane. Under the condition of nitrogen protection, the mixture was reacted at 85°C for 16 hours, concentrated to remove the solvent...
Embodiment 3
[0466]
[0467]
[0468] 1) Synthesis of intermediate Y: Add thieno[3,2-b]thiophene (140 mg, 1 mmol), N-bromosuccinimide (480 g, 0.9 mmol) and Dichloromethane 12 ml. Under the condition of nitrogen protection, the mixture was reacted at room temperature for 16 hours, 12 ml of water was added, extracted with dichloromethane (15 ml, 3 times) and concentrated to remove the solvent to obtain 180 mg of intermediate Y. Yield 82.1%. ESI-MS (M+H + ): 219.8.
[0469] 2) Synthesis of intermediate Z: Add compound Y (175.2 mg, 0.8 mmol), compound 3 (182.4 mg, 0.96 mmol), potassium carbonate (331 mg, 2.4 mmol), tetratriphenyl Phosphopalladium (92.6 mg, 0.08 mmol), THF 20 mL and water 6 mL. Under the condition of nitrogen protection, the mixture was reacted at 68°C for 16 hours, concentrated to remove the solvent, and then separated by silica gel column chromatography (petroleum ether) to obtain 170 mg of intermediate Z. Yield 74.8%. 1 H-NMR (400MHz, DMSO-d 6 )δ: 7.196(d,1H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com