Surface functionalized ordered mesopore nitrogen-doped carbon material and preparation method thereof
A surface functionalization and mesoporous technology, which is applied in the field of preparation of ordered mesoporous nitrogen-doped carbon materials, can solve the problems of low specific surface area and porosity, restricting applications, affecting the self-assembly process of carbon source and soft template, etc. The method is simple, the interaction is improved, and the effect of large application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Take melamine sulfate as precursor
[0040]Add 50mL of 0.2M sulfuric acid and 6g of F127 into a 100mL beaker, stir to dissolve. The mixed solution was transferred to a three-necked flask containing 1.26 g (0.01 mol) of melamine, and stirred and refluxed at 92° C. for 24 h. Add 5.4g (0.03mol) of fructose and continue to reflux for 10min. The obtained solution was transferred to a reaction kettle, placed in an oven at 130°C, and reacted for 3d. After the reaction, the product is suction filtered, washed with distilled water several times, and dried to obtain the corresponding carbon material. Then the material is calcined by the above two-step method (the specific steps of the two-step method are: the dried hydrothermal product is first calcined in air at 400°C for 2h to remove the soft template, and then calcined at 900°C for 1h) to obtain ordered mesoporous nitrogen-doped carbon material.
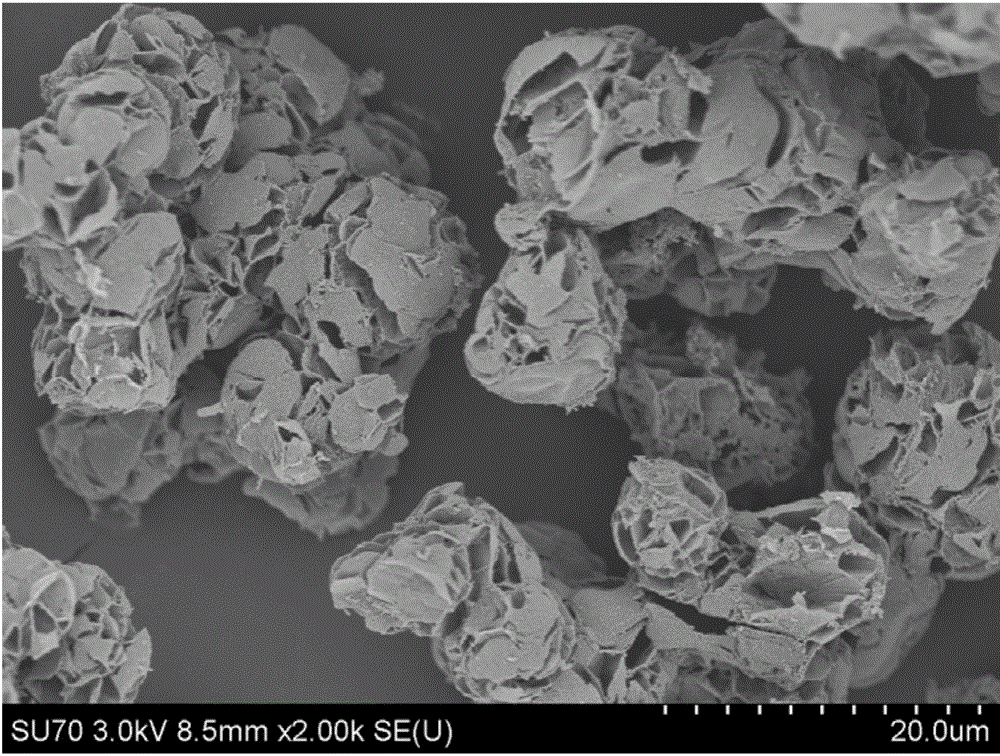
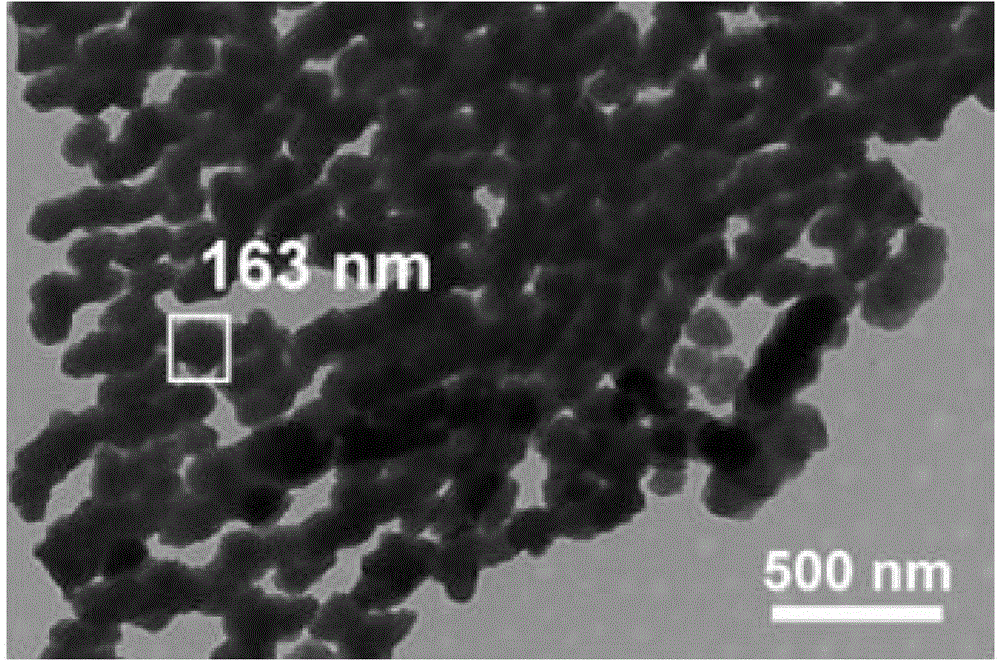
[0041] The scanning electron micrographs of the ordered mesopo...
Embodiment 2
[0050] Embodiment 2: Taking melamine phosphate as a precursor
[0051] Add 50mL of 0.2M phosphoric acid and 6g of F127 into a 100mL beaker, stir to dissolve. The mixed solution was transferred to a three-necked flask containing 1.26 g (0.01 mol) of melamine, and stirred and refluxed at 92° C. for 24 h. Add 5.4g (0.03mol) of fructose and continue to reflux for 10min. The obtained solution was transferred to a reaction kettle, placed in an oven at 130°C, and reacted for 3d. After the reaction, the product is suction filtered, washed with distilled water several times, and dried to obtain the corresponding carbon material. Then the material was calcined by the above-mentioned two-step method (same as Example 1) to obtain an ordered mesoporous nitrogen-doped carbon material. Its scanning electron microscope image (SEM) is shown in Figure 7(a), and its transmission electron microscope image (TEM) is shown in Figure 7(b).
Embodiment 3
[0052] Embodiment 3: Taking melamine oxalate as a precursor
[0053] Add 50mL of 0.1M oxalic acid and 6g of F127 into a 100mL beaker, stir to dissolve. The mixed solution was transferred to a three-necked flask containing 1.26 g (0.01 mol) of melamine, and stirred and refluxed at 92° C. for 24 h. Add 5.4g (0.03mol) of fructose and continue to reflux for 10min. The obtained solution was transferred to a reaction kettle, placed in an oven at 130°C, and reacted for 3d. After the reaction, the product is suction filtered, washed with distilled water several times, and dried to obtain the corresponding carbon material. Then the material was calcined by the above-mentioned two-step method (same as Example 1) to obtain an ordered mesoporous nitrogen-doped carbon material. Its scanning electron microscope image (SEM) is shown in Figure 8(a), and its transmission electron microscope image (TEM) is shown in Figure 8(b).
[0054] The ordered mesoporous nitrogen-doped carbon material ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com