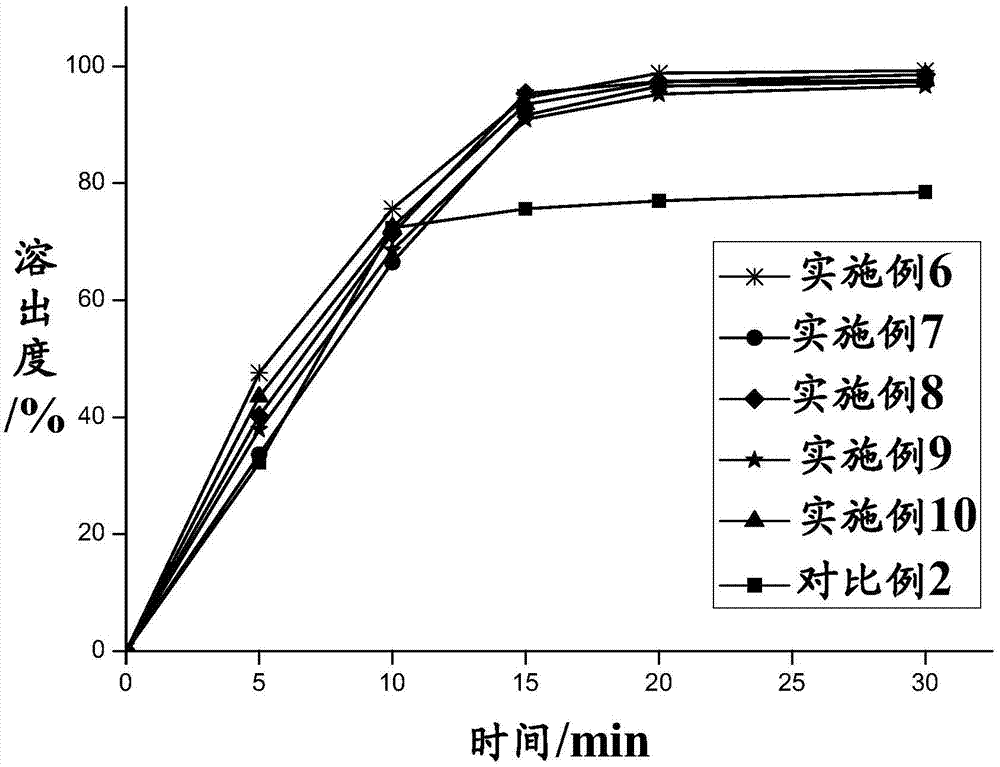
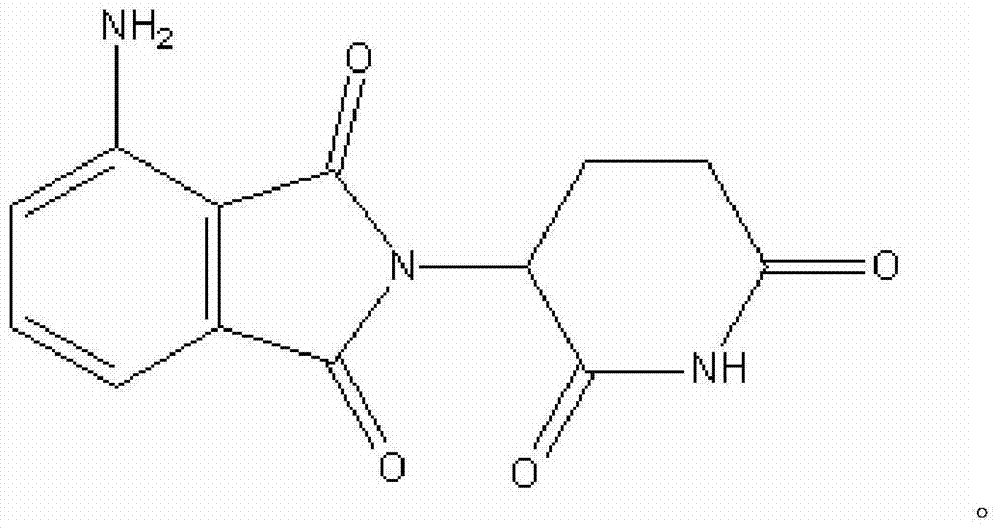
Pomalidomide nanoparticle and preparation and preparation method thereof
A pomalidomide and nanoparticle technology, which is applied in the field of medicine, can solve the problems of low pomalidomide solubility, drug particle size that cannot meet the drug onset, and increased specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a preparation method of pomalidomide preparation, comprising the following steps:
[0050] a) mixing pomalidomide, nanocarriers and a solvent to obtain a mixed liquid;
[0051] b) drying the mixed liquid to obtain pomalidomide nanoparticles;
[0052] c) mixing the pomalidomide nanoparticles with a diluent and a lubricant to obtain a pomalidomide preparation.
[0053] The invention mixes the pomalidomide, the nano carrier and the solvent to obtain the mixed liquid. In the present invention, the source of the pomalidomide is not particularly limited, and commercially available pomalidomide well known to those skilled in the art can be used. The pomalidomide preparation provided by the present invention includes 0.5 to 1.5 parts by weight of pomalidomide, preferably 1 part by weight.
[0054] In the present invention, the nanocarrier preferably includes lecithin, povidone, polyalkylcyanoacrylate, acrylamide, N,N-methylene diacrylate a...
Embodiment 1
[0064] (1) 45g soybean lecithin and 8g povidone K30 are dissolved in 50% ethanol aqueous solution, after adding 1g pomalidomide, place in high-speed stirring homogenizer, stir 60min with the stirring speed of 3000r / min, obtain mixed liquid;
[0065] (2) The obtained mixed liquid was spray-dried using a high-efficiency spray dryer, the spray speed was 5mL / min, the inlet air temperature was 60°C, and the outlet air temperature was 25°C to obtain pomalidomide nanoparticles.
Embodiment 2
[0067] (1) 30g soybean lecithin and 6g povidone K30 were dissolved in 50% ethanol aqueous solution, after adding 1g pomalidomide, placed in a high-speed stirring homogenizer, stirred for 60 minutes with a stirring speed of 3000r / min, get mixed liquid;
[0068] (2) The obtained mixed liquid was spray-dried using a high-efficiency spray dryer, the spray speed was 5mL / min, the inlet air temperature was 60°C, and the outlet air temperature was 25°C to obtain pomalidomide nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com