Protein-polymer combination and preparation method thereof
A technology for polymer conjugates and proteins, which is applied in the field of protein-polymer conjugates and their preparation, and can solve the problems of reduced biological activity, low reaction yield, and difficult control of binding sites and coupling stoichiometry.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
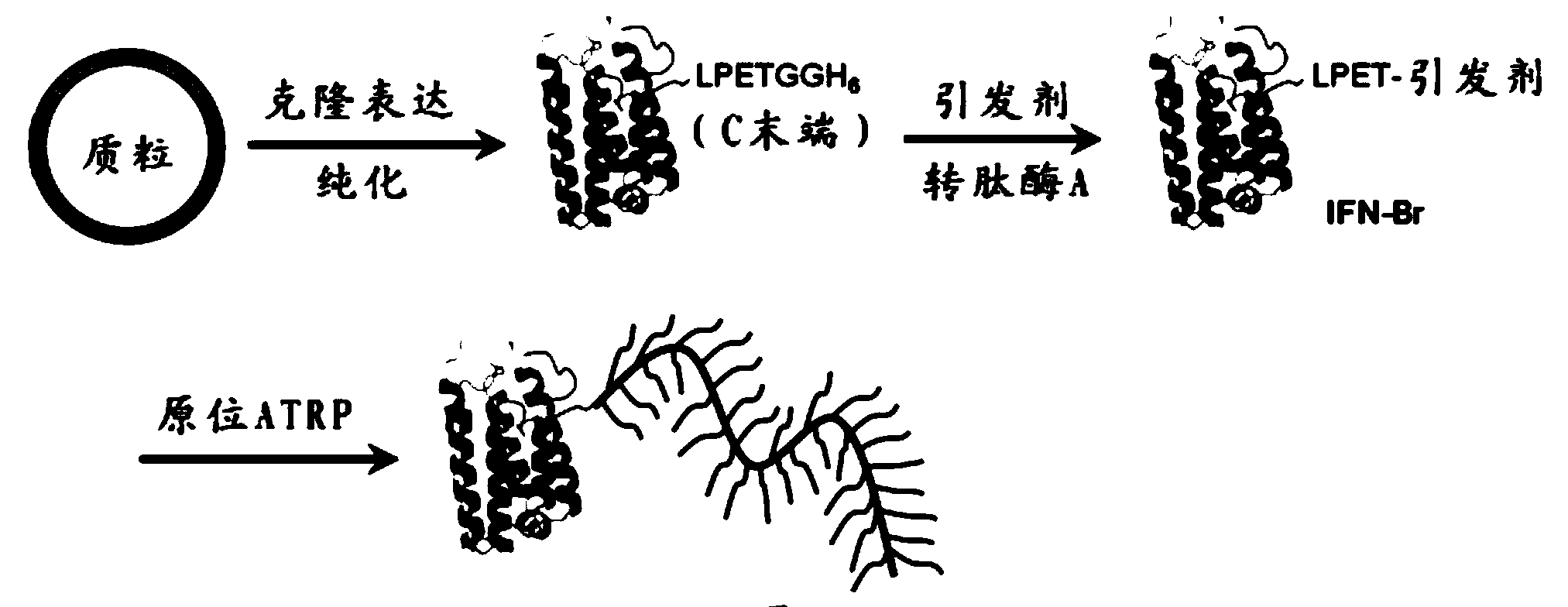
[0058] Example 1: Construction of IFN-LPETGGH6 fusion protein plasmid and plasmid expressing transpeptidase Sortase A-H6 and expression in Escherichia coli.
[0059] The sequence design of IFN-LPETGGH6 is as follows (see sequence 1 of the sequence listing):
[0060] GSGGGGS LP ETGGHHHHHH
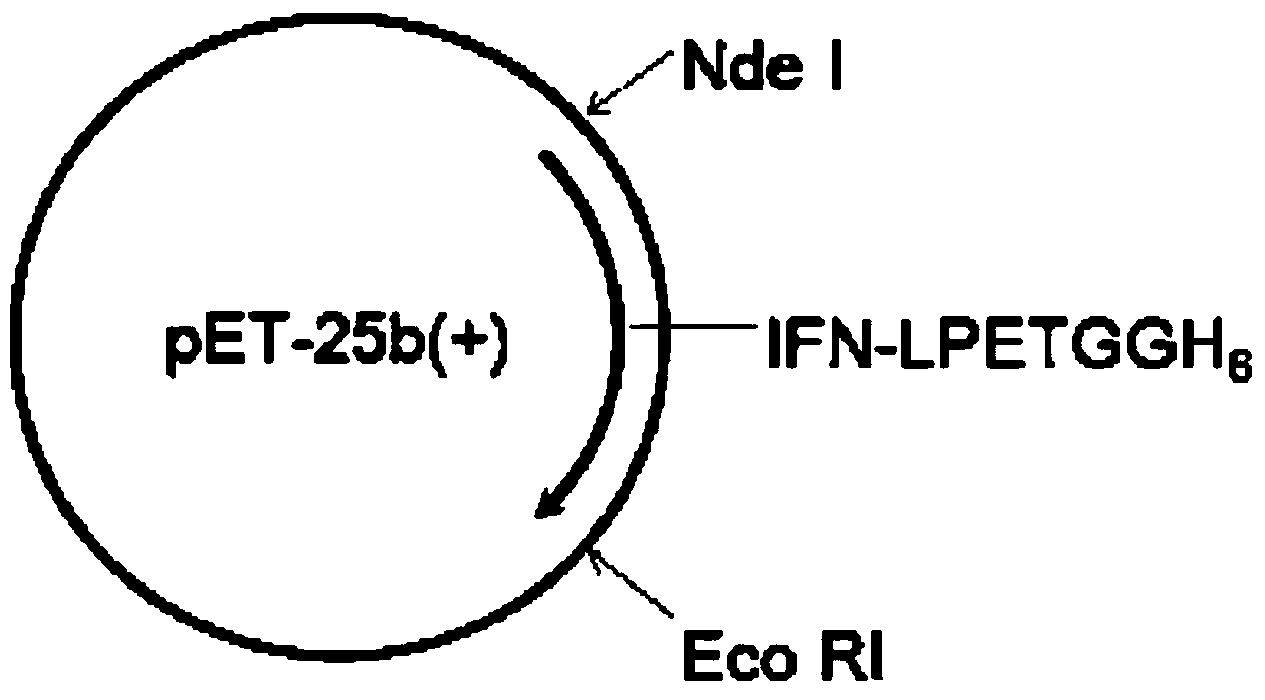
[0061] Among them, italics is the IFN sequence, LPETGGH6 is underlined and connected to IFN by GSGGGGS. The gene sequence was synthesized and inserted by Sangon Biotech (Shanghai, China) in the carrier. Using PCR technology, from Amplify the IFN-LPETGGH6 coding sequence in the vector, and insert it into the pET-25b (+) vector through Nde I and Eco RI restriction sites (such as figure 2 shown), the primers are as follows:
[0062] Upstream primer (see sequence 2 of the sequence listing):
[0063] 5' TTCCCCCATATGTGTGATCTGCCTCAGACTCATT 3'
[0064] Downstream primer (see sequence 3 of the sequence listing):
[0065] 5'TTCCCCGAATTCTTATCAATGATGATGATGATGGTGGCCACC 3'
[0066] Sub...
Embodiment 2
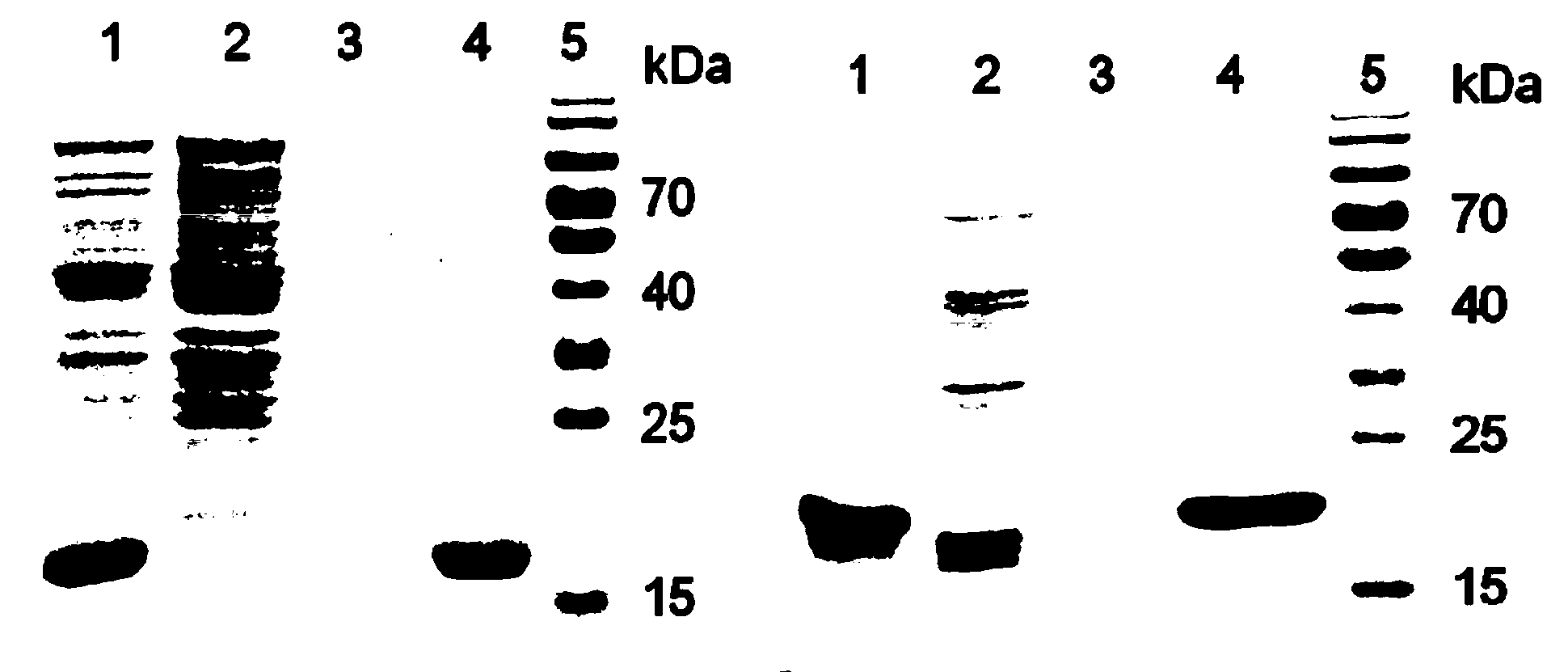
[0070] Embodiment 2: Purify IFN-LPETGGH by nickel affinity chromatography column 6 protein
[0071]Collect 1L of Escherichia coli culture solution in a centrifuge bottle, collect the bacteria by centrifugation at 3000×g, and remove the supernatant culture solution. The cells were resuspended in 30 mL of ice-cold PBS, and the cells were disrupted by an ultrasonic instrument at 4°C, and then the crushed E. coli products were centrifuged at 4°C and 14,000×g centrifugal force for 15 minutes. Add 2 mL polyethyleneimine (PEI, 10%) to the collected supernatant, and centrifuge again for 15 minutes, in order to remove nucleic acid and other negatively charged substances in the cell lysate. The obtained supernatant was filtered through a 0.45 μm filter membrane, and then purified on an AKTA protein purification system (AKTA Purifier 10, GE) through a nickel affinity chromatography column (HisTrap HP 5 mL), and the equilibration buffer was 10 mM PBS, 500 mM NaCl, 5% glycerol, 10mM imid...
Embodiment 3
[0073] Example 3: Introduction of ATRP initiator at the C-terminus of IFN by transpeptidase A enzyme catalysis
[0074] in the presence of IFN-LPETGGH 6 (200μM) in 50mM Tris·HCl solution (10mL) was added 5mM 2-(2-(2-(2-aminoacetamido)acetamido)acetamido)ethyl 2-bromo-2-methylpropane Ester (initiator AEBM such as Figure 4 indicated), with 100 μM transpeptidase A and 20 mM CaCl 2 Tris·HCl solution (10 mL) was mixed and reacted overnight at room temperature. Figure 5 shows that the ATRP initiator is attached to the C-terminus of IFN catalyzed by transpeptidase A, Figure 5 Among them, cysteine (Cys) of transpeptidase A acts as a nucleophilic group to attack, acting on IFN-LPETGGH 6 The peptide bond between the threonine and glycine of the upper recognition sequence LPETG breaks it (acylation), thereby generating an acylase intermediate, and then the amino group of the triglycine nucleophilicly attacks the acylase, covalently linking AEBM to the on IFN-α2. After the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com