A dendritic gene drug carrier and its preparation and application
A dendritic and product technology, applied in gene therapy, drug combination, anti-tumor drugs, etc., can solve the problems of cytotoxicity and poor degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of the carrier material 8armPEG-SS-PEG-Dendrimer G2 (PSPG2s) and carrying drugs and genes. (Dendrimer is AB3-Dendrimer G2, 8armPEG is 15000Da, NHS-PEG-OH is 2000Da, linker is CDI, drug is doxorubicin, gene is blank reporter gene DNA)
[0080] (1) AB 3 - Synthesis of Dendeimer G2
[0081] Weigh 445.6 mg of compound 1 and dissolve it in 15 ml of dichloromethane, add 871 mg of 1-hydroxy-7-azobenzotriazole (HOAt) and 3 ml of triethylamine, stir at room temperature for 10 minutes, then add 2.1 g of N-tert-butoxycarbonylethylenediamine, then slowly add 4.05 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) at 0°C After the addition, keep the temperature at 0°C for 1 hour, and then react at room temperature for 4 hours. After the reaction was completed, 30 milliliters of ethyl acetate was added to the reaction solution, and 20 milliliters of the organic phase was washed three times with 0.4 mol / liter hydrochloric acid, three time...
Embodiment 2
[0097] Example 2: Characterization of materials
[0098] (1) AB 3 -Characterization of Dendrimer G2
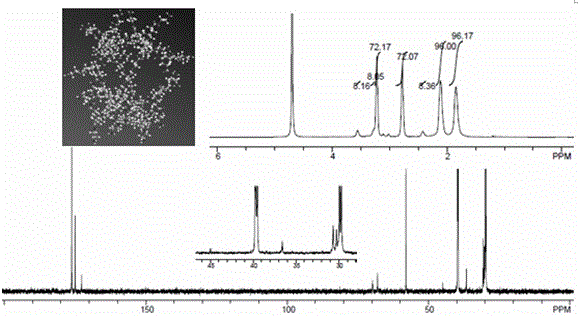
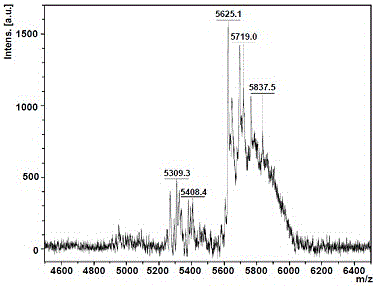
[0099] figure 2 for AB 3 -H NMR and C NMR spectra of Dendrimer G2, image 3 for AB 3 -The high-resolution mass spectrum (MALDI-TOF MS) spectrum of Dendrimer G2 is basically consistent with the theoretical molecular weight of 5604, indicating that AB 3 - The preparation method of Dendrimer G2 is feasible.
[0100] (2) Synthesis and characterization of 8armPEG-SS-PEG-Dendrimer G2
[0101] Figure 4 It is eight-branched polyethylene glycol (8arm-PEG), eight-branched polyethylene glycol (8arm-PEG-SS-PEG) linked by disulfide bonds to straight-chain polyethylene glycol, and the second generation of AB 3 type dendrimers (AB 3 -Dendrimer G2), the final product dendritic polycation material (8armPEG-SS-PEG-Dendrimer G2, PSPG2s) H NMR spectrum, the displacement of each inactive hydrogen atom in the figure is shown in the figure, the NMR of the final product PSPG2s The hydroge...
Embodiment 3
[0102] Example 3: Ability of Compounds to Carry Genes
[0103] Image 6 (a) is Dendrimer G2 / DNA and PSPG2s / DNA with 4 access ratios (PSPG2s-2 / 4 / 6 / 8 respectively represent the molar ratio of 8arm-PEG and Dendrimer G2 in the dendritic polycation compound PSPG2s is 2 / 4 / 6 / 8) Retardation test of agarose gel electrophoresis carrying DNA at different N / P ratios. The figure shows that compounds such as PSPG2s can effectively carry DNA, and the efficiency is higher than that of Dendrimer G2 that exists alone. Figure (b) is the agarose gel electrophoresis retardation test of PSPG2s-2 / 4 / 6 / 8 carrying RNA under different N / P ratios. The figure shows that compounds such as PSPG2s can also effectively carry RNA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com