Biological material for composite fibroblast growth factor 1 and application of biological material in preparation of medicines for treating lower limb ischemia
A fibroblast and growth factor technology, applied in cardiovascular system diseases, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as high cost, achieve easy acceptance, promote functional recovery, and improve motor function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
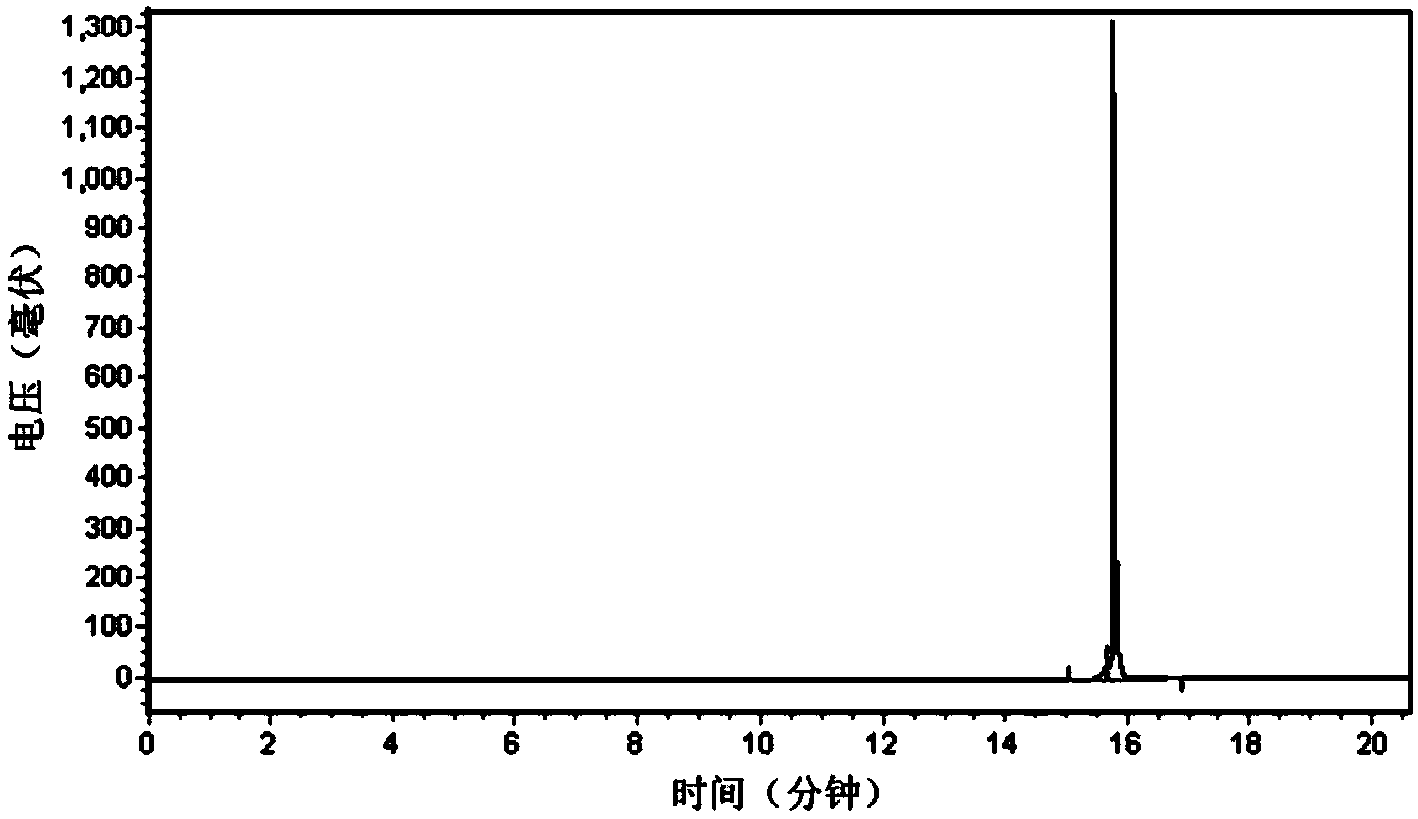
[0027] Embodiment 1: Synthesis of RAD16-II polypeptide
[0028] 1. Materials and equipment
[0029] 1.1 Reagents
[0030] Fluorenylmethoxycarbonyl (Fmoc)-amino acid is a product of SIAM in the United States; PyBOP, Boc-Ala-Merrifield resin, hexahydropyridine, and lutidine are products of Merck; dimethylformamide (DMF) is a product of Samsung, Japan Products (soaked in ninhydrin and Molecular sieve dehydration, and determination of no free amino groups); dichloromethane (DCM) is a product of China Pharmaceutical (Group) Shanghai Chemical Reagent Company (soaked with anhydrous potassium carbonate before use); trifluoroacetic acid (TFA) is a product of GEEL BELGIUM company Methanol is a product of Shanghai Zhenxing Chemical Factory No. 1; HPLC methanol is a product of Merck; tetrahydrofuran is a product of Shanghai Chemical Reagent Station Central Chemical Factory.
[0031] 1.2 Instruments
[0032] The 431A peptide synthesizer is the product of Applied biosystems; the high p...
Embodiment 2
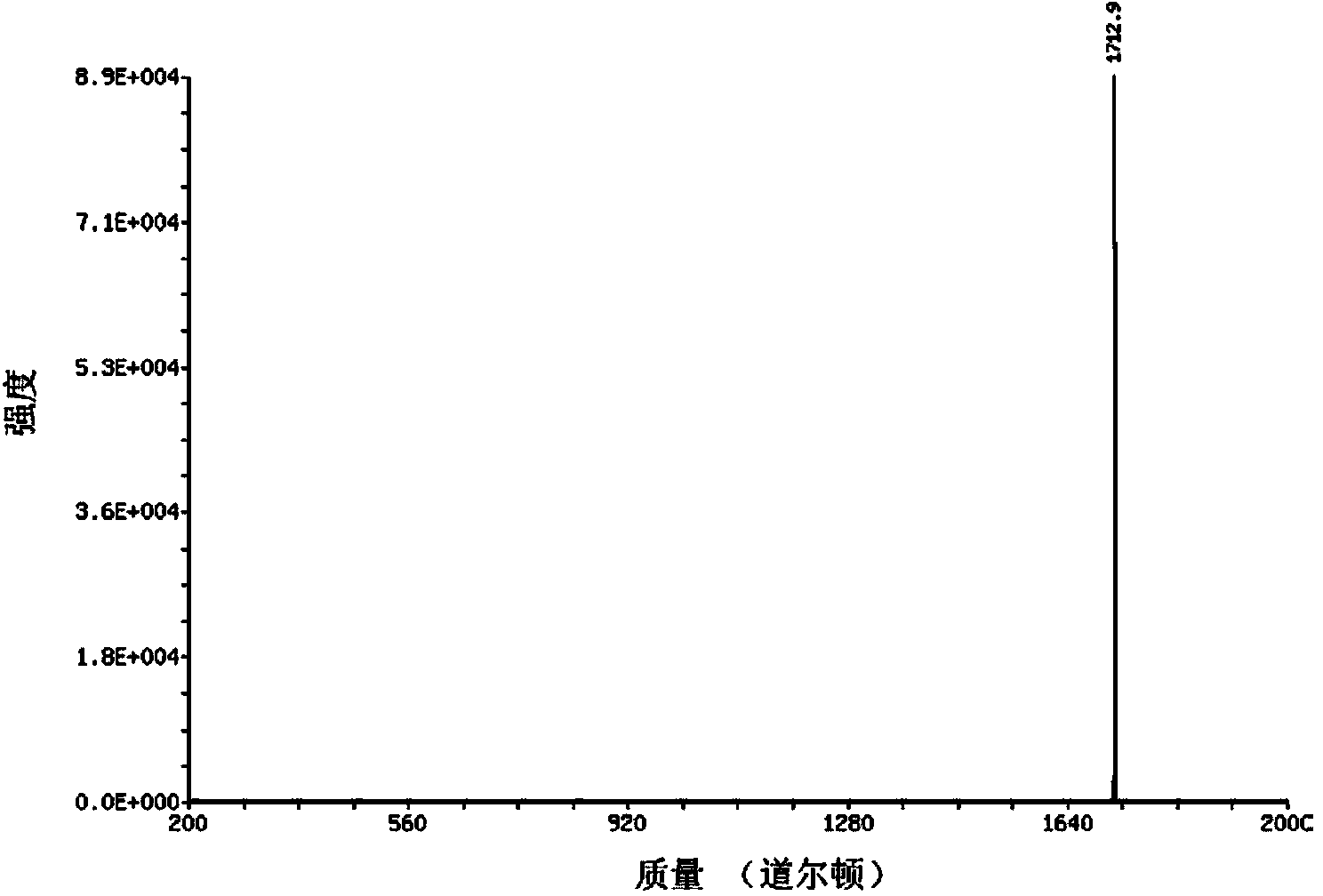
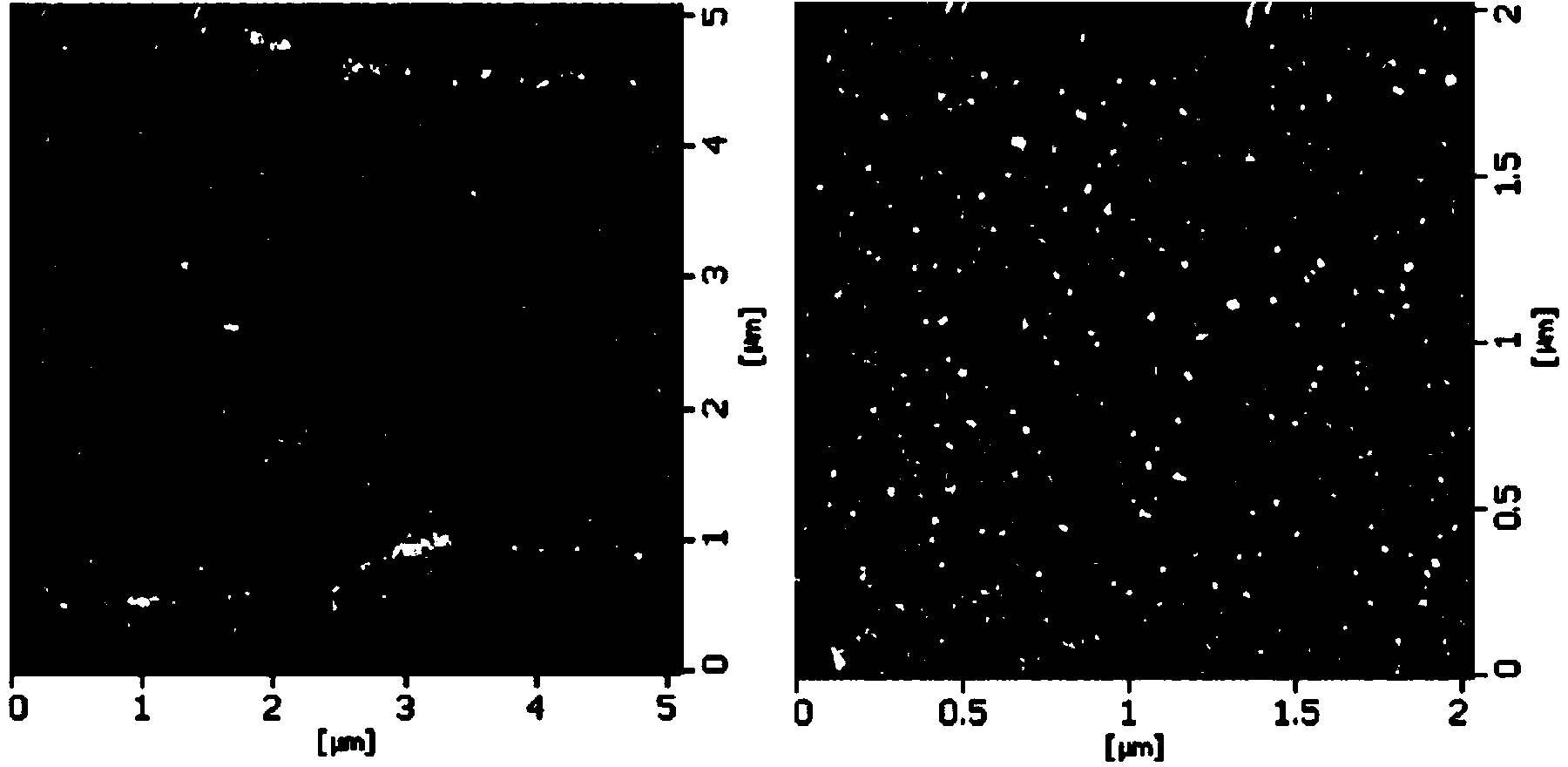
[0060] Embodiment 2: the preparation of the biological material of composite fibroblast growth factor 1 (FGF1)
[0061] 1. Reagents and equipment
[0062] 1.1 Reagents: RAD16-II polypeptide prepared in Example 1; FGF1 purchased from R&D Company; sucrose solution.
[0063] 1.2 Instruments: ultra-clean table; ultrasonic instrument; 4 ℃ refrigerator.
[0064] 2. Preparation method
[0065] (1) Sterilization of deionized water
[0066] Take 500ml of deionized water (18MΩ) in the pure water system (Millipore Milli-Q system), and use the standard process of autoclaving to sterilize, that is, treat the deionized water at a vapor pressure of 103.4kPa and a temperature of 121.3°C for 30 Minutes, and then sterilized deionized water was sealed and stored in a refrigerator at 4°C for later use;
[0067] (2) Prepare sucrose sterile solution
[0068] At room temperature and normal pressure, the sucrose was prepared into a sucrose solution with a concentration of 100 mg / mL with the deio...
Embodiment 3
[0073] Embodiment 3: the preparation of the biological material of composite fibroblast growth factor 1 (FGF1)
[0074] 1. Reagents and equipment
[0075] 1.1 Reagents: RAD16-II polypeptide prepared in Example 1; FGF1 purchased from R&D Company; sucrose solution.
[0076] 1.2 Instruments: ultra-clean table; ultrasonic instrument; 4 ℃ refrigerator.
[0077] 2. Preparation method
[0078] (1) Sterilization of deionized water
[0079] Same as the sterilization treatment of deionized water in Example 2.
[0080] (2) Prepare sucrose sterile solution
[0081] With the preparation of sucrose solution among the embodiment 2.
[0082] (3) Preparation of raw material solution
[0083] Dissolve the RAD16-II polypeptide and FGF1 prepared in the embodiment in the aseptic environment of the ultra-clean bench at room temperature and normal pressure in the sucrose solution sterilized in step (2) to form a mixed solution. In the mixed solution, RAD16 The concentration of -II polypeptide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com