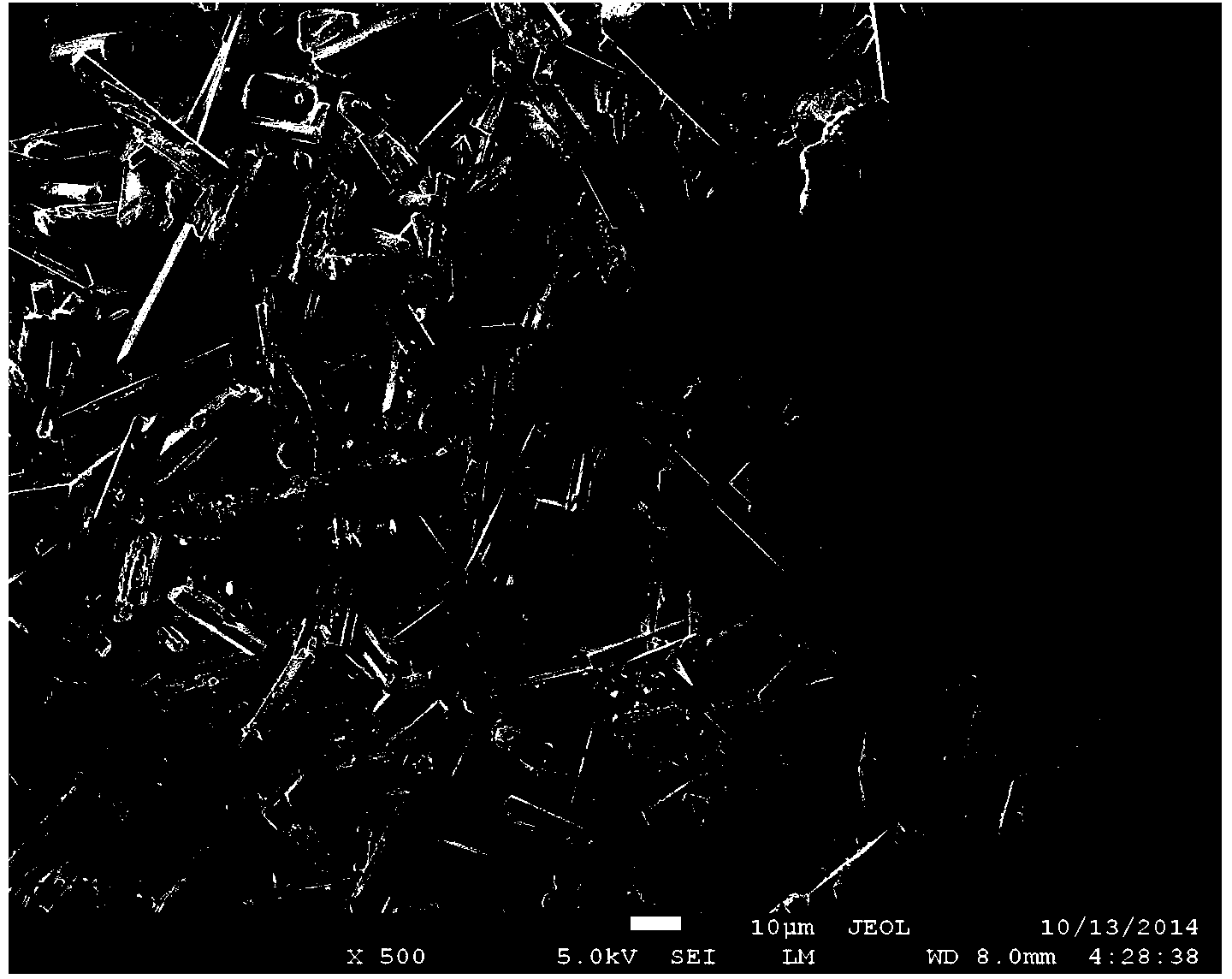
Preparation method of calcium sulfate whiskers
A technology of calcium sulfate whiskers and calcium sulfate, applied in the field of fillers, can solve the problems of environmental pollution, low efficiency, high energy consumption, etc., and achieve the effects of high reaction efficiency, low energy consumption, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1), preparation of calcium sulfate particles
[0031] D 50 Particles of 2 to 25 μm.
[0032] (2) Preparation of stabilizer
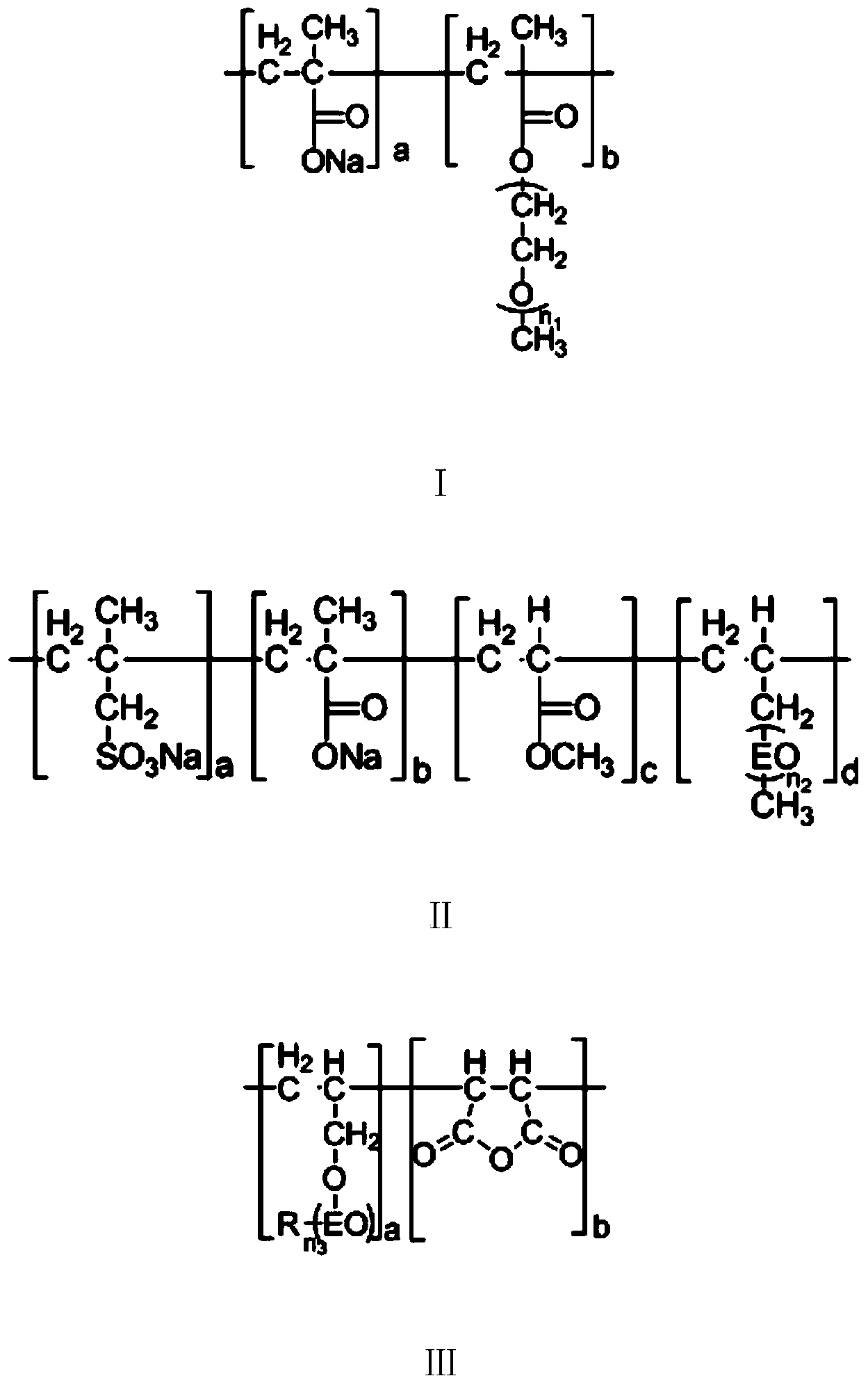
[0033] Add 180g water, 150gPEG (molecular weight is 2000) in the glass reactor, pass N under stirring 2 Substitution, the temperature was raised to 60° C., and 0.2 g of benzoyl peroxide was added. 15 g of methacrylic acid, 24.5 g of methyl methacrylate and 3 g of sodium methallyl sulfonate were added dropwise within 4 hours. Simultaneously, 40 g of an aqueous solution containing 0.3 g of ascorbic acid and 0.2 g of sodium edetate was added dropwise, and kept at 60° C. for 4 hours to complete the reaction. Adjust the pH of the reaction solution to 7 with a 30% sodium hydroxide aqueous solution, cool, filter with suction, and dry in vacuum to obtain a stabilizer. The structural formula of the stabilizer is shown in Formula II.
[0034] (3) Preparation of calcium sulfate whiskers
[0035] The calcium sulfate particle of 172g, the stabilizing ag...
Embodiment 2
[0037] (1), the preparation of calcium sulfate particle is with embodiment 1.
[0038] (2) Preparation of stabilizer
[0039] In glass reactor, add 170g water, 151gPEG (molecular weight is 2000), pass N under stirring 2 Replacement, warming up to 65°C, adding 17.05g of H 2 o 2 (mass concentration is 2%). 13.5 g of methacrylic acid, 24 g of methyl methacrylate and 5 g of sodium methallylsulfonate were added dropwise within 5 hours. Simultaneously, 40 g of an aqueous solution containing 0.2 g of vitamin E and 0.2 g of potassium persulfate was added dropwise and kept at 60° C. for 3 hours to complete the reaction. Use 30% sodium hydroxide aqueous solution to adjust the pH of the reaction solution to 7, cool, filter with suction, and dry to obtain a stabilizer. The structural formula of the stabilizer is shown in formula II.
[0040] (3) Preparation of calcium sulfate whiskers
[0041] The calcium sulfate particle of 172g, the stabilizing agent of 0.35g and the water of 800g...
Embodiment 3
[0043] (1), the preparation of calcium sulfate particle is with embodiment 1.
[0044] (2) Preparation of stabilizer
[0045] In the glass reactor, add 175g water, 158gPEG (molecular weight is 2000), pass N under stirring 2 replacement, warming up to 65°C, adding 14g of H 2 o 2 (mass concentration is 2%). 11 g of methacrylic acid, 19 g of methyl methacrylate, 4.5 g of sodium methallylsulfonate and 35 g of allyl polyethylene glycol (50) ether were added dropwise within 4 hours. Simultaneously, 38 g of an aqueous solution containing 0.3 g of ascorbic acid and 0.35 g of sodium edetate was added dropwise, and kept at 60° C. for 4 hours to complete the reaction. Adjust the pH of the reaction solution to 7 with a 30% sodium hydroxide aqueous solution, cool, filter with suction, and dry to obtain a stabilizer. The structural formula of the stabilizer is shown in Formula I.
[0046] (3) Preparation of calcium sulfate whiskers
[0047] The calcium sulfate particle of 172g, the st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com