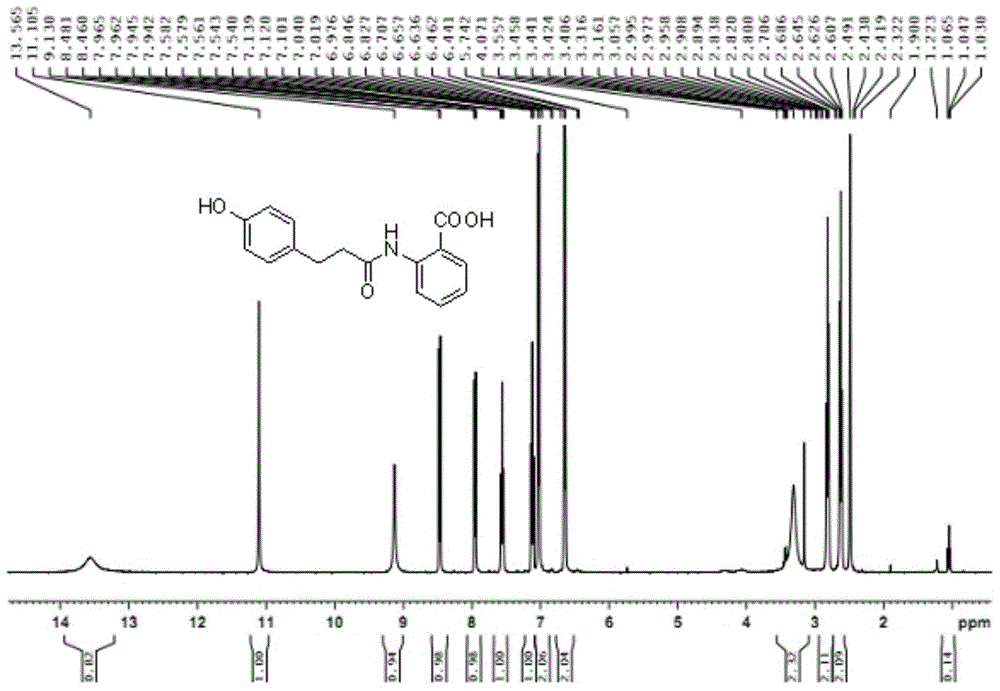
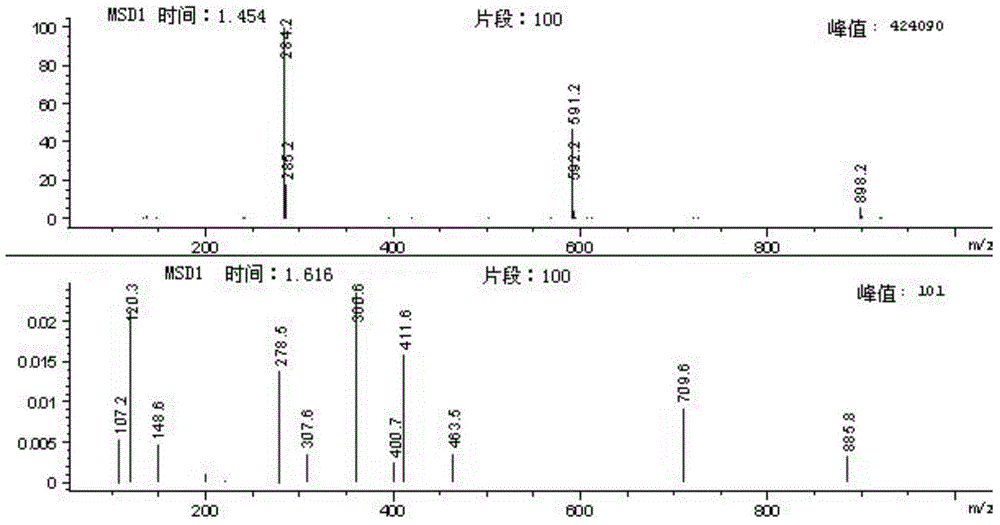
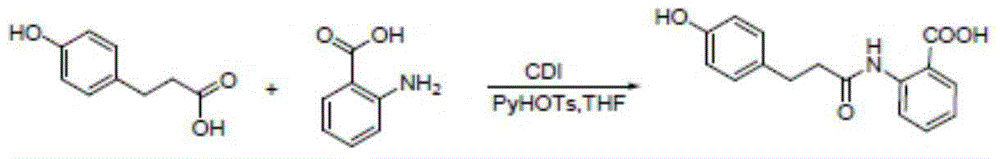
Method for synthesizing 4-hydroxy benzenepropionamido benzoic acid
A technology of avenyl o-aminobenzoic acid, which is applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of emission and high production cost, and achieve emission avoidance, pollution reduction, and labor intensity reduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Add p-hydroxyphenylpropionic acid (3 g, 0.018 mol), DMF (0.25 ml, 0.0036 mol) and dichloromethane (100 ml) into a single-neck bottle, and stir at 18°C at room temperature. Thionyl chloride (2ml, 0.027mol) was added dropwise to the suspension and stirred at room temperature for about 3 hours. The system changed from slightly muddy to a clear yellow solution. Continue to add methyl anthranilate (2.7g, 0.018mol). ), after the dripping, a large amount of white solid precipitated, and then sodium carbonate (3g, 0.0283mol) was added, the system became very viscous, after 3 hours of reaction, TLC spot plate (take a little sample, add water and ethyl acetate, organic Layer point board, develop solvent ethyl acetate: petroleum ether=1:5 (V / V)), generate a main point. After 4 hours, the plate was turned on. The raw materials were reduced, but the solution was acidic. Add 1 g of sodium carbonate to continue the reaction. Extend the reaction time overnight (12 hours), TL...
Embodiment 2
[0036] Add p-hydroxyphenylpropionic acid (4.58 g, 0.03 mol), DMF (0.4 ml, 0.003 mol), and dichloromethane (50 ml) into a single-neck bottle, and stir at room temperature or in a cold water bath (10°C). Thionyl chloride (3.6ml, 0.05mol) was added dropwise to the suspension, stirred at room temperature for about 12 hours, the system changed from slightly muddy to a clear yellow solution, and methyl anthranilate (4.53g, 0.03mol) ), after the dripping, a large amount of white solid precipitated out. Sodium hydroxide (5.28g, 0.132mol) was added in batches. After 12 hours of reaction, the pH value was weakly alkaline. The methylene chloride was recovered by rotary evaporation to obtain a solid. Add water 30 ml each of methanol and methanol, and add sodium hydroxide solution (2.14g, 0.066mol) with a concentration of 10mol / l, keep the liquid in the single-neck bottle at pH 12, stir at room temperature for about 2 hours, the system is milky white, add concentrated hydrochloric acid Adju...
Embodiment 3
[0038] Add p-hydroxyphenylpropionic acid (3.0 g, 0.018 mol), DMF (0.25 ml, 0.0036 mol) and dichloromethane (50 ml) into a single-neck flask, and stir at 18°C. Thionyl chloride (2ml, 0.027mol) was added dropwise to the suspension, stirred at room temperature for about 0.5 hours, the system changed from slightly muddy to a clear yellow solution, and methyl anthranilate (2.7g, 0.018mol) was added dropwise After the dripping, a large amount of white solid precipitated. Add disodium hydrogen phosphate trihydrate (4.93g, 0.0823mol) in batches. After 12 hours of reaction, the dichloromethane was recovered by rotary evaporation to obtain a solid. Add 30 ml of water and 30 ml of ethanol each , And add sodium hydroxide solution (2.14g, 0.066mol) with a concentration of 10mol / l, keep the pH of the liquid in the single-neck bottle at 12, stir at room temperature for about 24 hours, the system is milky white, add concentrated hydrochloric acid to adjust the pH to 6 , A white solid precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com