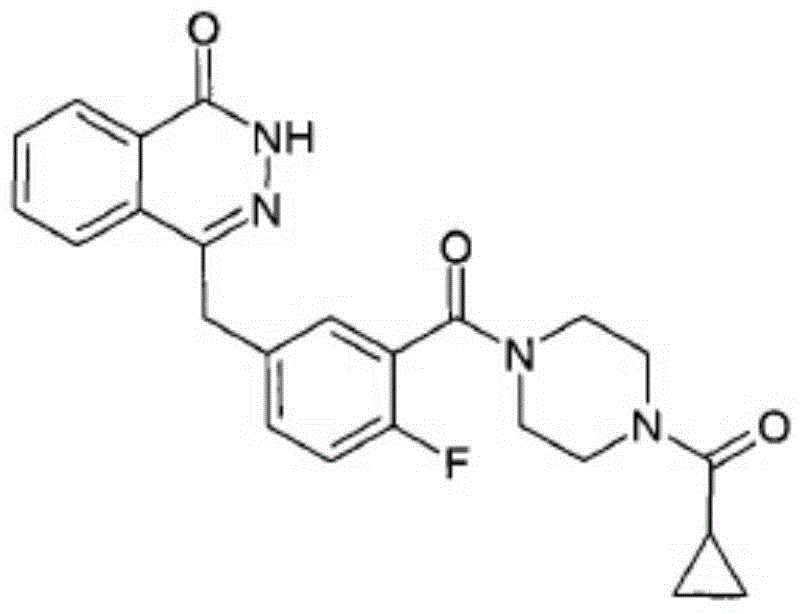
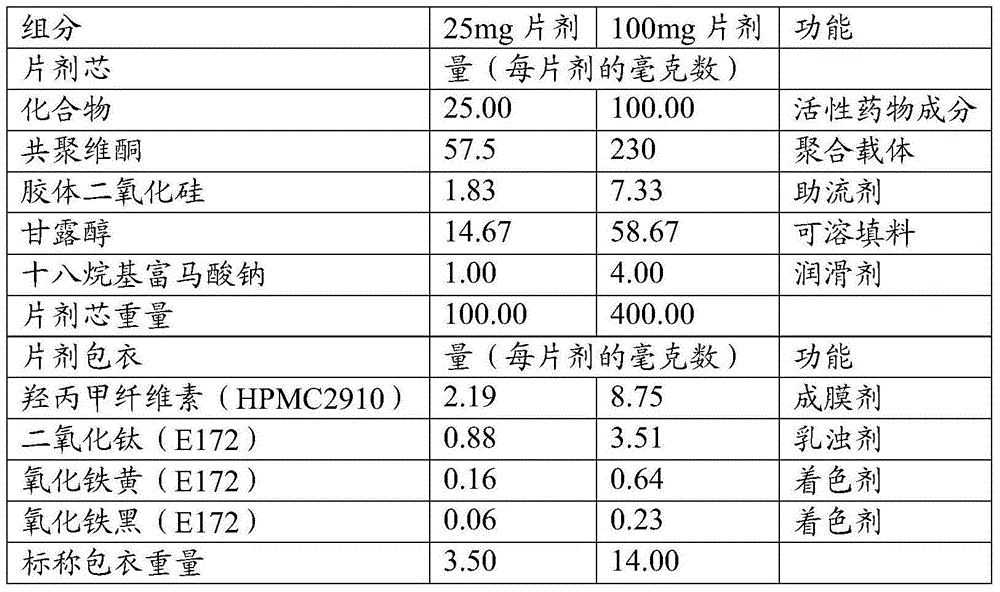
Olaparib solid dispersion preparation and preparation method thereof
A solid dispersion and tablet technology, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc., can solve the impact of the stability of olaparib preparations, narrow access, lack of Safety and other issues, to achieve the effect of ensuring the dissolution effect, bioavailability and stability, which is conducive to industrial production and has a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] According to the preparation scheme of the present invention, those skilled in the art can choose the melt extrusion method, spray drying method and solvent evaporation method that have been matured in the art to prepare the olaparib solid dispersion, and can also be prepared according to the three methods described in the present invention. A kind of preparation scheme is prepared, and the method is as follows:
[0054] 1. Melt extrusion method
[0055] Take the prescribed amount of olaparib and povidone, add part of the lubricant, mix well, and put the mixture in a twin-screw extruder to extrude the mixture. During the extrusion process, apply a vacuum to the extrusion barrel to remove the melt The extrudate is calendered by passing it through two counter-rotating calender rolls, followed by cooling before grinding to obtain a solid dispersion.
[0056] 2. Spray drying method
[0057] Dissolve the Olaparib and povidone in the prescribed amount in an organic solvent ...
Embodiment 1
[0061] Embodiment 1: Olaparib solid dispersion tablet
[0062] prescription:
[0063] components
mg / tablet
olapani
25
Povidone K30
125
Microcrystalline Cellulose pH102
40
8
1
1
Sheet weight
200
[0064] Preparation process: solvent evaporation method
[0065] Dissolve the prescribed amount of Olaparib and Povidone K30 in a solvent of acetone:methanol (1:3), volatilize under reduced pressure in a water bath at 60°C, vacuum degree 0.07-0.08MPa, and recover organic matter under reduced pressure. After the solvent becomes viscous, continue vacuum drying under reduced pressure for 1 hour, transfer to a vacuum drying oven, dry at 40°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0066] Add the prepared solid dispersion to the prescription amount of microcrystalline cellulos...
Embodiment 2
[0067] Embodiment 2: Olaparib solid dispersion tablet
[0068] prescription:
[0069] components
mg / tablet
olapani
25
[0070] Povidone K30
75
Microcrystalline Cellulose pH102
60
Crospovidone
6
1
2
Sheet weight
169
[0071] Preparation process: solvent evaporation method
[0072] Take Olaparib and povidone K30 in the prescribed amount, dissolve them in methanol:dichloromethane (4:1) solvent, put them in a water bath at 60°C, vacuum 0.07-0.08MPa, recover the organic solvent under reduced pressure, wait for After becoming viscous, continue vacuum drying under reduced pressure for 1 hour, transfer to a vacuum drying oven, dry at 40°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0073] The prepared solid dispersion is added into the prescribed amount of microcrystalline cellulose pH102, cros...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com