Near-infrared fluorescent dye with large stokes shift and synthesis method and application
The technology of a fluorescent dye and a synthesis method, applied in the field of specific fluorescence detection, can solve the problems of self-quenching of the dye and the reduction of the fluorescence intensity of the dye, and achieve the effects of easy purification, stable fluorescence intensity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
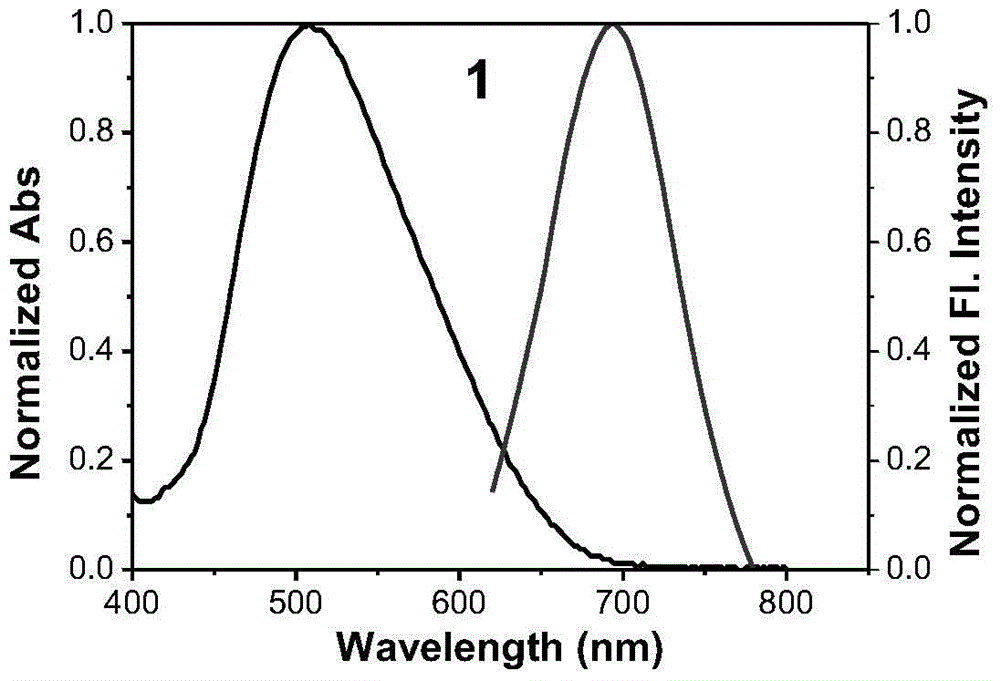
[0037] The synthesis of embodiment 1 fluorescent dye
[0038] Synthesis of Compound 1:
[0039] (1) Synthesis of intermediate c:
[0040] Get raw materials a and b (the amount of a and b added is 1mmol) (indole iodine salt a and condensing agent b are prepared with reference to the prior art, references: Tang, B.; Yu, F.; Li, P. ; Tong, L.; Duan, X.; Xie, T.; Wang, X.J.Am Chem.Soc.2009,131,3016-3023.), dissolved in 20ml of toluene-acetic acid mixture (volume ratio of toluene and acetic acid 15:5), reacted at 110°C for 2h; after the reaction was completed, concentrated under reduced pressure at 60°C for 30min, removed the solvent, and carried out column separation using a thin-layer chromatographic column (the stationary phase in the chromatographic column was silica gel). The eluent is a dichloromethane-methanol mixture, and in the mixture, the volume ratio of dichloromethane to methanol is 50:1; a black-red solid is obtained, which is intermediate c (60% yield);
[0041] (...
Embodiment 2
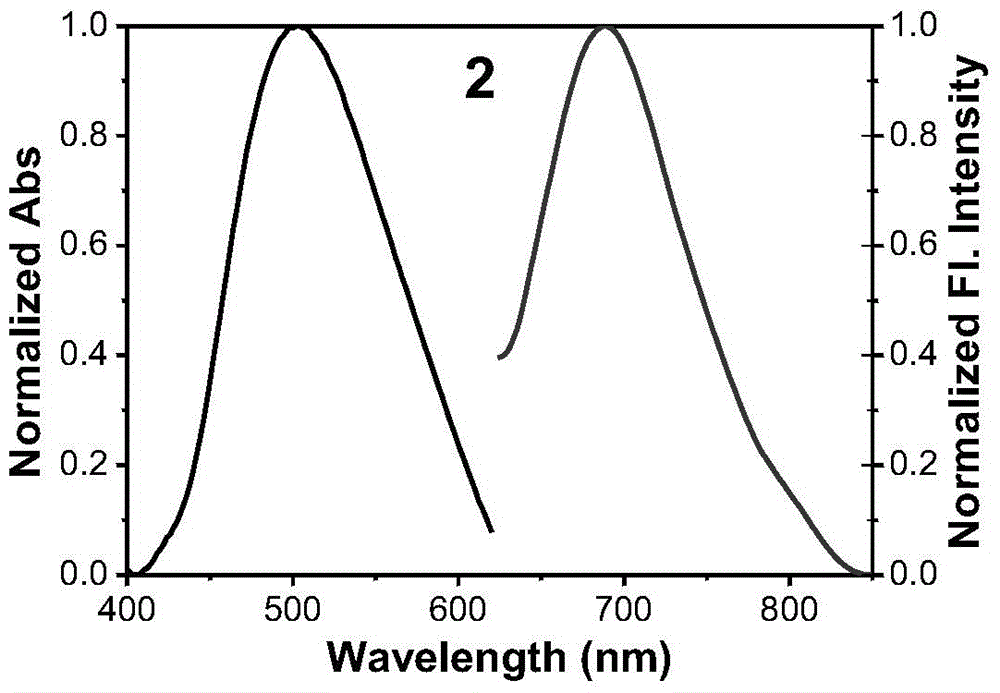
[0047] The synthesis of embodiment 2 fluorescent dyes
[0048] Synthesis of Compound 2:
[0049] The synthetic method of Compound 2 is the same as that of Compound 1 in Example 1, except that the raw material in step (2) is changed from acetophenone to 4-methylacetophenone. Fluorescent dye compound 2 (R is Me) is a black-red solid with a yield of 68%.
[0050] Concrete synthetic route is as follows:
[0051]
[0052] The infrared, NMR and mass spectrometry of compound 2 are characterized as follows:
[0053] Compound 2: IR:v C=O =1639.25cm -1 ,v C-H =2926.57cm -1 . 1 H NMR (600MHz, CDCl 3 ):1.26(t,J=6.0Hz,4H),1.69(m,6H),1.87(s,3H),5.50(q,1H),6.65(d,J=2.4Hz,1H),6.90(t ,J=6.0Hz,1H),6.95(d,J=12.0Hz,1H),7.17(t,J=6.0Hz,2H),7.25(t,J=5.4Hz,7H),7.62(d,J =12.0Hz,1H),7.84(d,J=6.0Hz,3H),7.89(d,J=12.0Hz,3H). 13 C NMR (150MHz, CDCl 3 ):11.1,21.3,21.6,26.2,27.3,28.0,28.1,29.3,29.7,36.9,46.2,92.6,106.2,109.9,120.2,121.7,124.3,127.2,127.7,128.4,128.8,129.1,136.9 139.3, 140.9, ...
Embodiment 3
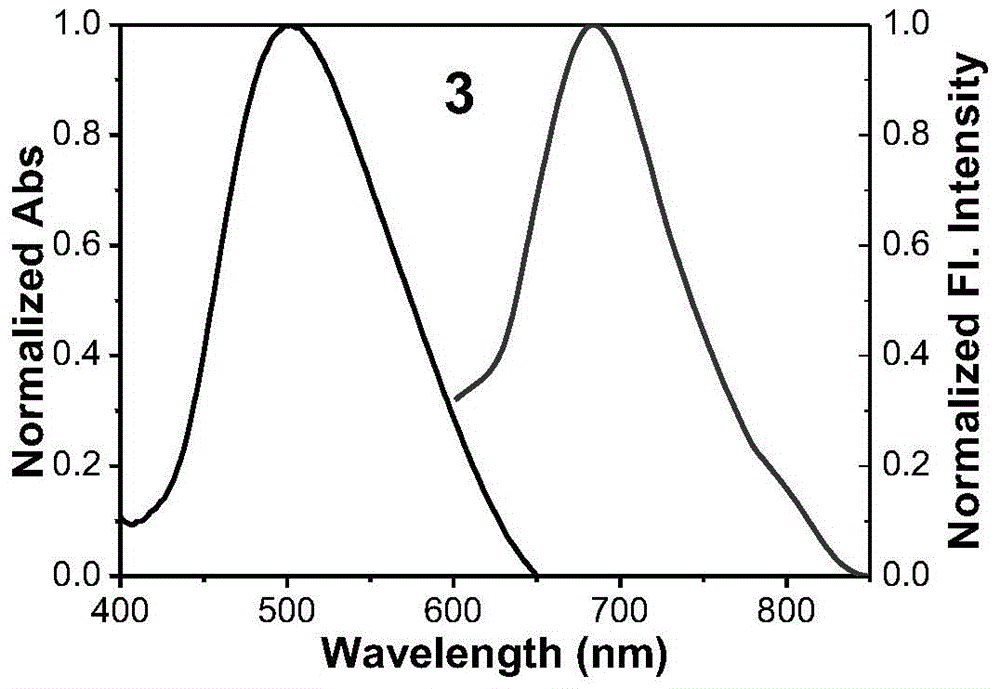
[0054] Example 3 Synthesis of fluorescent dyes
[0055] Synthesis of compound 3:
[0056] The synthetic method of Compound 3 is the same as that of Compound 1 in Example 1, except that the raw material in step (2) is changed from acetophenone to 4-methoxyacetophenone. Fluorescent dye compound 3 is a black-red solid with a yield of 74%.
[0057] The synthetic route is as follows:
[0058]
[0059] The infrared, NMR and mass spectrometry of compound 3 are characterized as follows:
[0060] Compound 3: IR:v C=O =1672.52cm -1 ,v C-H =2934.26cm -1 ,v C-H =2839.52cm -1 . 1 H NMR (600MHz, CDCl 3 ):1.25(t,J=6.0Hz,3H),1.64(s,6H),1.87(t,J=6.0Hz,2H),2.57(m,4H),3.70(m,2H),3.85(m ,3H),5.48(d,J=12.0Hz,1H),6.64(d,J=7.8Hz,1H),6.80-6.90(m,4H),7.18(t,J=7.8Hz,2H),7.61 (d,J=13.8Hz,1H),7.99(d,J=9.0Hz,2H),8.34(d,J=15.0Hz,1H). 13 C NMR (150MHz, CDCl 3 ):11.1,21.3,26.2,27.3,28.1,30.0,36.9,46.2,55.43,55.46,92.6,106.2,110.0,113.6,119.9,120.2,121.2,124.3,127.2,127.7,128.6,1390.2,5, 140...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com