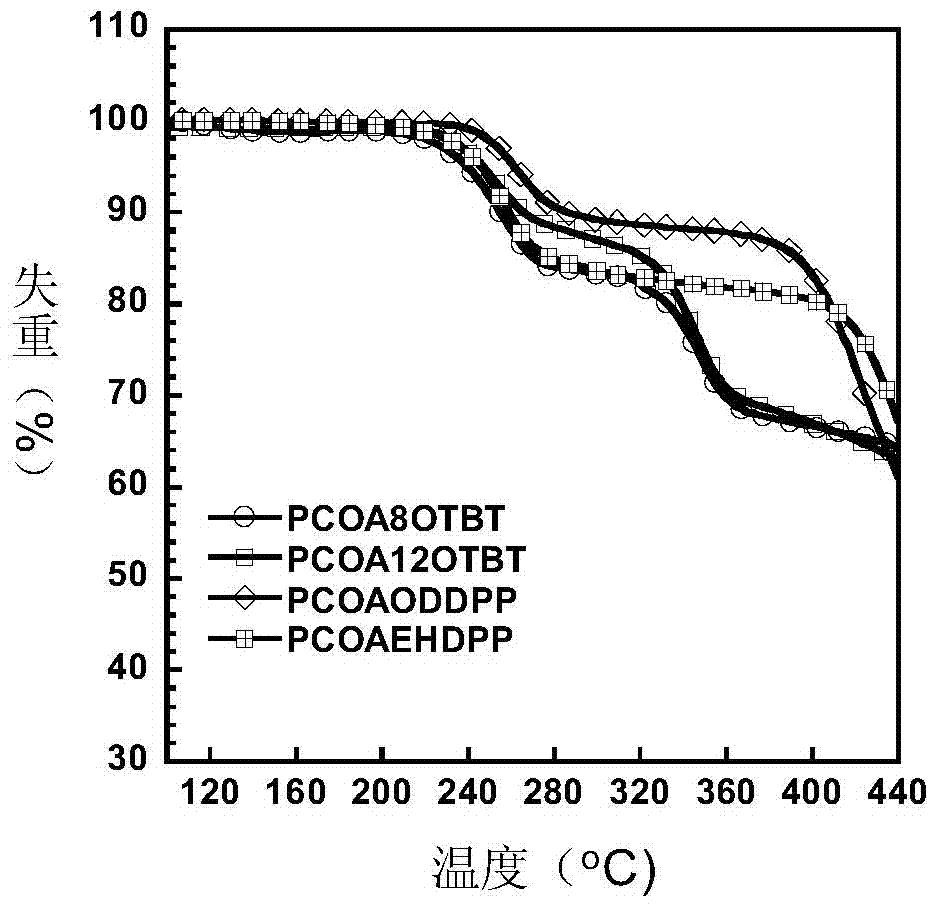
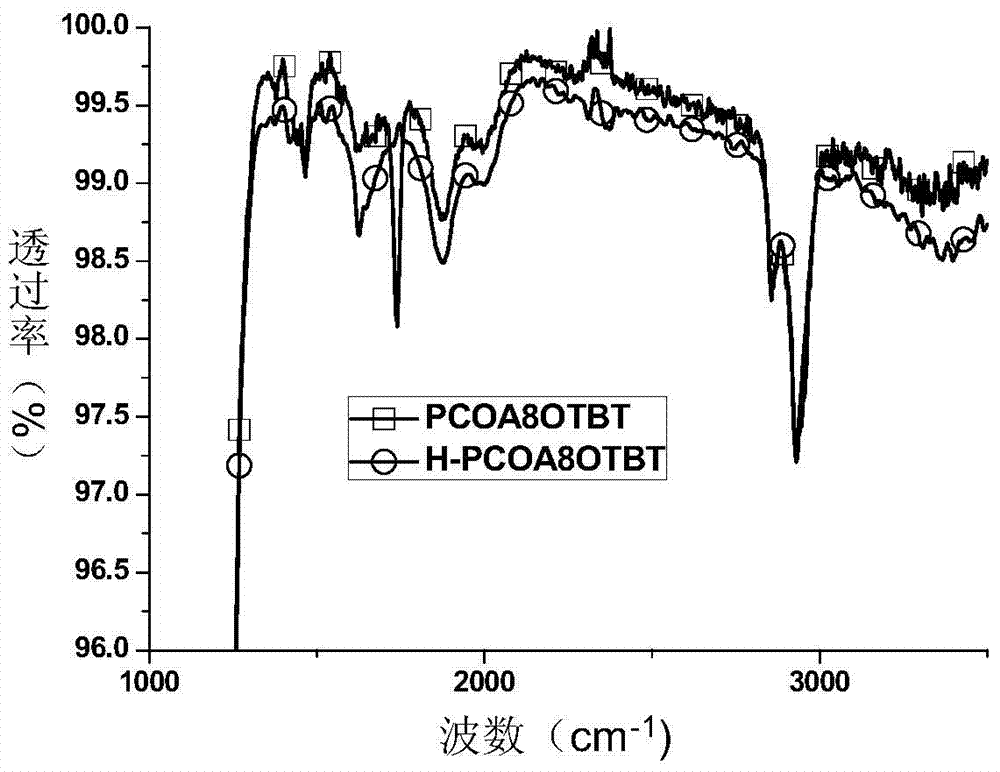
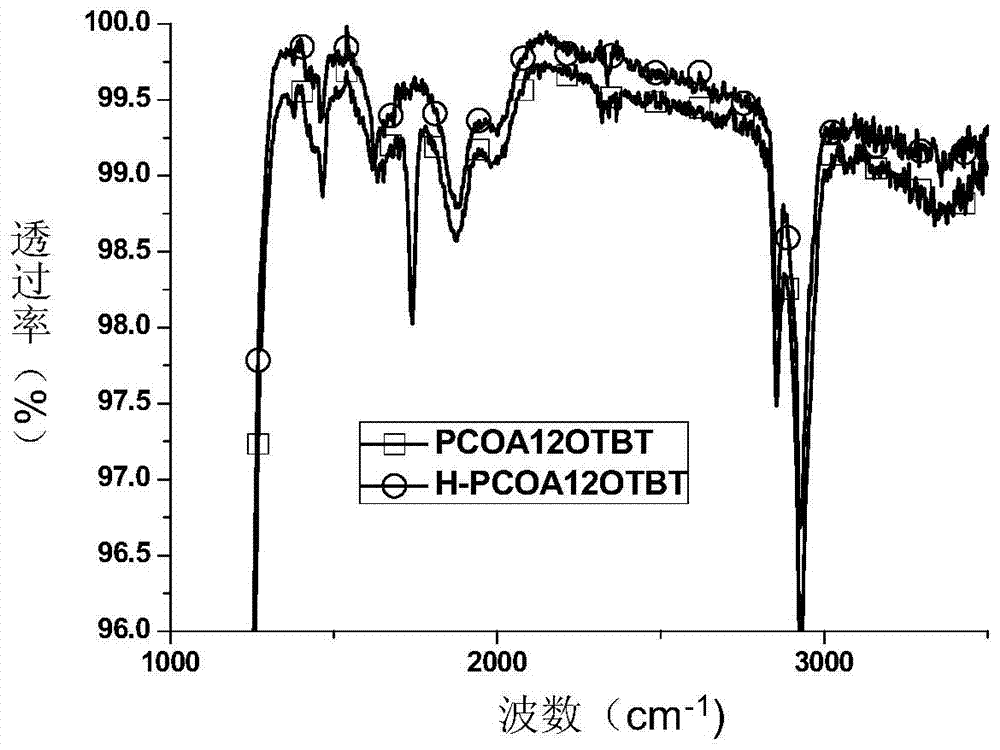
Donor-receptor type organic semiconductor material with removable group anthracene unit and preparation method of donor-receptor type organic semiconductor material
A technology of organic semiconductors and groups, applied in the field of donor-acceptor organic semiconductor materials and preparation, can solve the problems of planarity damage of conjugated units, reduce the performance of organic semiconductor devices, reduce the charge transport capacity of materials, etc., and achieve good results. Photoelectric properties, excellent charge transport properties, good solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of Bilateral Boronate Products with Removable Group Anthracene Units 3
[0057] The synthetic route is as follows:
[0058]
[0059] (1) Synthesis of Compound 1
[0060] 40 mL of toluene was added to 2,6-dibromoanthracene (3.36 g, 10 mmol) and maleic anhydride (3.43 g, 35 mmol), and the reaction solution was heated to reflux for 24 hours. Cooled to room temperature, the reaction solution was extracted with ethyl acetate, the obtained organic phase was dried with anhydrous magnesium sulfate, and the solvent was removed by a rotary evaporator to obtain a viscous liquid product, which was directly used in the next reaction without separation and purification.
[0061] (2) Synthesis of compound 2
[0062] Compound 1 (4.66 g, 10 mmol) was dissolved in 20 mL of methanol, about 1 mL of concentrated sulfuric acid was added thereto, and the reaction solution was heated and refluxed for 24 hours. Cooled to room temperature, extracted with ethyl acetate, the obta...
Embodiment 2
[0067] Preparation of Polymer PCOA8OTBT Based on Removable Group Anthracene
[0068] The synthetic route is as follows:
[0069]
[0070] Compound 4 was synthesized with reference to literature (Macromolecules, 2010, 43, 9771.)
[0071] Compound 3 (57.4 mg, 0.1 mmol), compound 4 (71.4 mg, 0.1 mmol) and two drops of trioctylmethylammonium chloride were added to a 25 mL round bottom flask and charged with nitrogen for 20 minutes. Palladium acetate (4 mg) and tris(4-methoxyphenyl)phosphine (20 mg) were added to the reaction flask, and nitrogen gas flow was continued for 20 minutes. Aqueous sodium carbonate (2M, 1 mL) and toluene (4 mL) were added to the reaction flask. The reaction solution was heated to 110°C and reacted for 4 hours, cooled to room temperature, and the reaction was dropped into methanol for precipitation, filtered to obtain a solid, which was extracted with acetone, n-hexane, and chloroform by a Soxhlet extractor in turn, and the chloroform was partially co...
Embodiment 3
[0081] Preparation of Polymer PCOA12OTBT Based on Removable Group Anthracene
[0082] The synthetic route is as follows:
[0083]
[0084] Compound 5 was synthesized with reference to literature (Macromolecules, 2010, 43, 9771.)
[0085] Compound 3 (57.4 mg, 0.1 mmol), compound 5 (82.7 mg, 0.1 mmol) and two drops of trioctylmethylammonium chloride were added to a 25 mL round bottom flask and charged with nitrogen for 20 minutes. Palladium acetate (4 mg) and tris(4-methoxyphenyl)phosphine (20 mg) were added to the reaction flask, and nitrogen gas flow was continued for 20 minutes. Aqueous sodium carbonate (2M, 1 mL) and toluene (4 mL) were added to the reaction flask. The reaction solution was heated to 110°C and reacted for 4 hours, cooled to room temperature, and the reaction was dropped into methanol for precipitation, filtered to obtain a solid, which was extracted with acetone, n-hexane, and chloroform by a Soxhlet extractor in turn, and the chloroform was partially c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com