Method for separating and purifying protease capable of degrading soybean protein allergens
A soybean protein, separation and purification technology, applied in the field of protein purification, can solve the problems of few enzymes, separation and purification, etc., and achieves the effects of good stability, mild conditions and easy control of the method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 protease activity assay
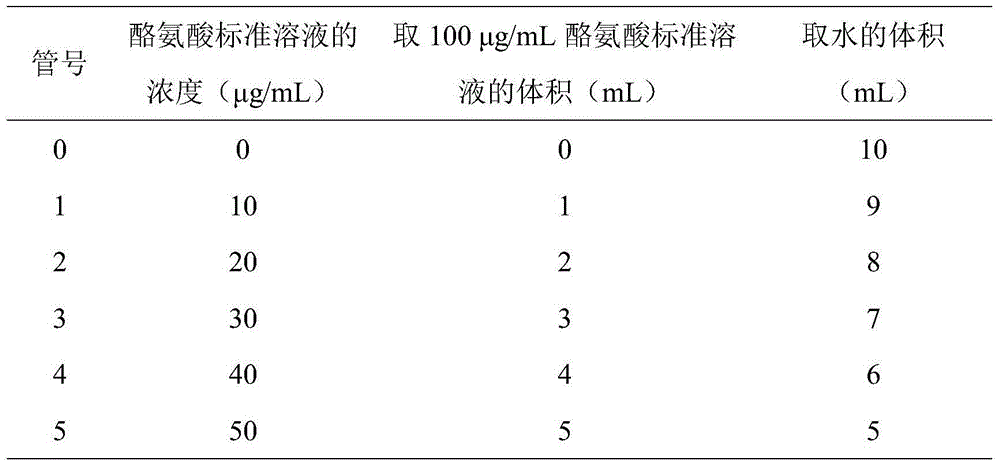
[0032] 1. L-tyrosine standard curve drawing: L-tyrosine standard solution is prepared according to Table 1
[0033] Table 1
[0034]
[0035]Take 1.00mL of each of the above solutions (parallel tests are required), add 5.0mL of 0.4mol / l sodium carbonate solution, and 1.00mL of Folin’s reagent solution, place in a water bath at 50±0.2°C for 20 minutes to develop color, and use a spectrophotometer At a wavelength of 660nm, in a 5mm cuvette, take the 0 tube without tyrosine as the blank, measure the absorbance respectively, and draw the standard curve with the absorbance A as the ordinate and the concentration of tyrosine as the abscissa.
[0036] 2. Determination of protease activity
[0037] Take 4 test tubes and number them 1, 2, 3, 4. Test tube No. 1 is the control. 2% casein was dissolved in PBS (sodium dihydrogen phosphate-disodium hydrogen phosphate) at pH 8.0, and then preheated at 50°C. First, add the preheated enzyme...
Embodiment 2
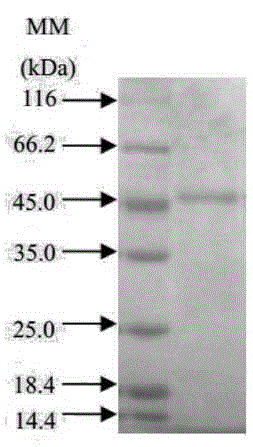
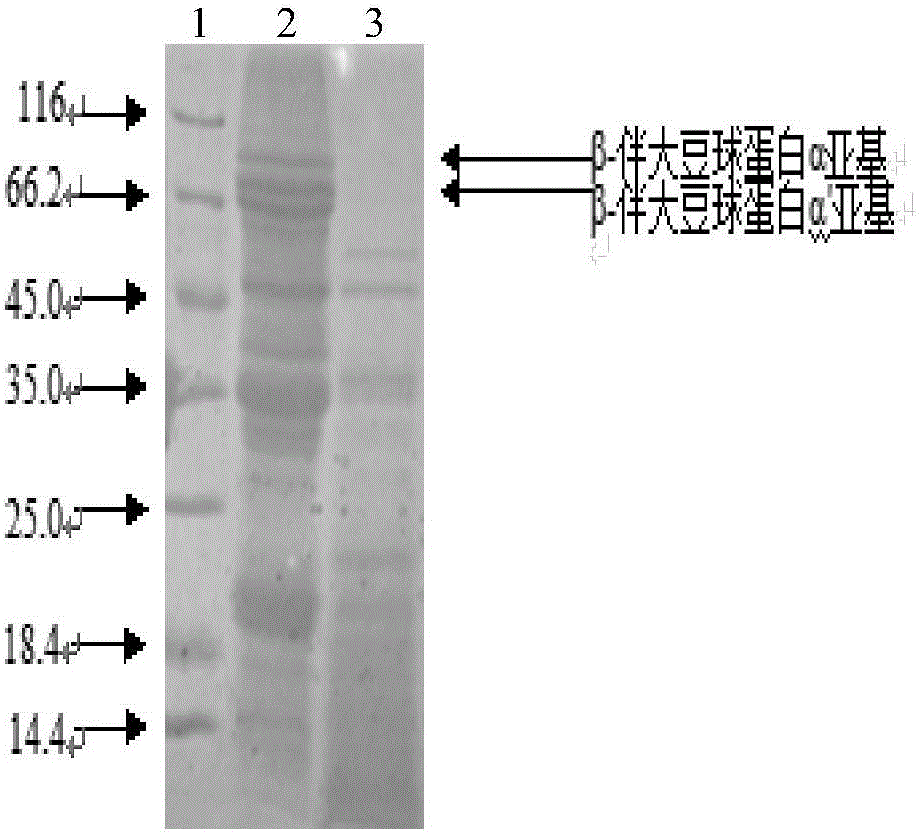
[0038] The purification of embodiment 2 protease
[0039] The fermentation broth was centrifuged at 7000rpm at 4°C for 20min to obtain the fermentation supernatant, and ammonium sulfate powder was added slowly at 4°C until the saturation of ammonium sulfate in the fermentation supernatant reached 50%, and the supernatant was collected after centrifugation at 7000rpm at 4°C for 20min, and the Ammonium sulfate was used to make the final concentration reach 80% saturation, and after standing at 4° C. for 1 h, the precipitate was collected by centrifugation at 4° C. at 7000 rpm. And the precipitated protein was dissolved in Tris-HCl buffer containing 25mmol / L, pH 9.0. Purified by anion exchange chromatography, the elution conditions are: equilibrium solution: 25mmol / L Tris-HCl buffer, pH 9.0; eluent: equilibrium solution containing 1mol / L NaCl; linear elution, flow rate is 1mL / min, Collect the protein eluted by about 15% of the eluent as the target protease solution, and then use...
Embodiment 3
[0042] Enzymatic properties of embodiment 3 protease
[0043] The enzymatic properties of the purified protease were studied, and it was found that the stability of the purified protease was better at pH 7.0-9.0 and 30-40°C. More than 80% of the enzyme activity; the pure enzyme is incubated at 30-40°C for 60 minutes, the stability is good, and the enzyme activity can still maintain more than 80%. Ca 2+ In the presence of 2+ , Fe 2+ and Cu 2+ It has obvious inhibitory effect on this protease; phenylmethylsulfonyl fluoride (PMSF) has a more significant inhibitory effect on this protease, but dithiodinitrobenzoic acid (DNTB) has no inhibitory effect on this protease, so it is speculated that it is a serine protease. K of the protease m 4.19g / L, V max 4.04g / (L·s), K cat 107.73s -1 , K cat / K m It is 25.71L / (g·s).
[0044] 1 Optimum reaction pH and temperature of enzyme
[0045] (1) Optimum reaction pH: the purified proteases were tested for their enzyme activity in dif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com