Vaccine composition for preventing and controlling porcine reproductive and respiratory syndrome and having broad-spectrum mucosal immunity function and application of vaccine composition
A vaccine composition and a technology for respiratory syndrome, applied in the field of medicine and biology, can solve the problems of aggravation, affect the recovery of the disease, unclear mechanism, etc., achieve the improvement of the immune effect, reduce the risk of allergy and disease transmission, and prolong the immune period. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
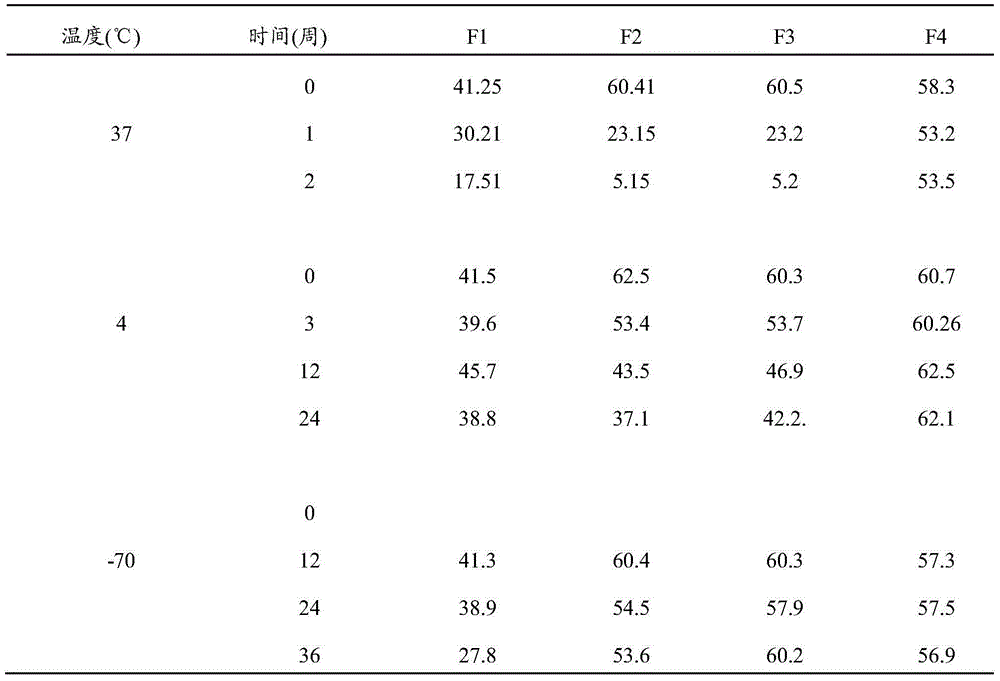
[0033] Example 1 Inactivation and Purification of Complete Porcine Reproductive and Respiratory Syndrome Virus Antigen and Preparation of Vaccine Composition
[0034] 1. Preparation of porcine reproductive and respiratory syndrome virus antigen
[0035] 1.1 Virus culture
[0036] MARC-145 cells were adapted to serum-free medium, transferred to a bioreactor filled with VP SFM medium (Invitrogen) and microcarrier cytodex 1, and cultured at 37°C for 3-4 days, the pH of the culture medium was 7.2±0.2, and oxygen saturation The temperature is 25±0.1%, and the stirring speed is medium speed. The porcine reproductive and respiratory syndrome virus was infected at 0.01 MOI, and the virus culture fluid was harvested at 7, 11, and 15 days, and the virus fluid was added to infect each harvest.
[0037] 1.2 Clarification
[0038] The harvested liquid was filtered with 8 μm polypropylene pre-filter, and then filtered and clarified through 0.8 μm and 0.2 μm filters, and the virus titer (...
Embodiment 2
[0058] The preparation of embodiment 2 lyophilized purified inactivated vaccines
[0059] 1. Preparation of porcine reproductive and respiratory syndrome virus antigen
[0060] Method is with embodiment 1.
[0061] 2. Preparation of stable synergist (100ml)
[0062] Method is with embodiment 1.
[0063] 3. Vaccine composition preparation
[0064] The obtained porcine reproductive and respiratory syndrome virus whole virus particle antigen is added to the prepared stable synergist, so that the amount of virus protein reaches 25 μg / mL.
[0065] Follow the steps below to lyophilize, freeze at -40°C, and sublimate twice, the first sublimation conditions: temperature -15°C, pressure 80μPa, until the ice is completely sublimated; the second sublimation conditions: temperature 40°C, pressure 80μPa, keep until residual The water content is 2-3%, and the virus loss is not more than 5% during the freeze-drying process. Ready to serve.
Embodiment 3
[0066] The preparation of embodiment 3 lyophilized purified inactivated vaccines
[0067] 1. Preparation of porcine reproductive and respiratory syndrome virus antigen
[0068] Method is with embodiment 1.
[0069] 2. Preparation of stable synergist (100ml)
[0070] To 100ml of 50mM phosphate buffer, add 1g of arginine and glutamic acid mixture in equal weight ratio, 2.0g of Poly ICLC, 8g of fucose, 5g of mannitol, 0.4g of urea, 0.04 gEDTA, 0.01g Tween-20, and adjust the pH value to 8.0.
[0071] 3. Vaccine composition preparation
[0072] The obtained porcine reproductive and respiratory syndrome virus whole virus particle antigen is added to the prepared stable synergist, so that the amount of virus protein reaches 25 μg / mL.
[0073] Follow the steps below to lyophilize, freeze at -40°C, and sublimate twice, the first sublimation conditions: temperature -15°C, pressure 80μPa, until the ice is completely sublimated; the second sublimation conditions: temperature 40°C, press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com