Vanadium-phosphorus oxide and preparation method thereof
A technology of vanadium phosphorus oxide and concentrated phosphoric acid, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., to achieve the effects of easy addition or removal, continuous preparation process, and improved reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
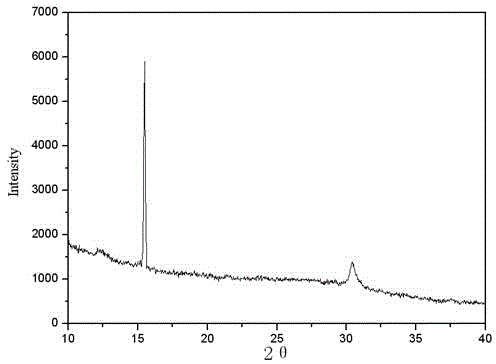
Image
Examples
Embodiment 1
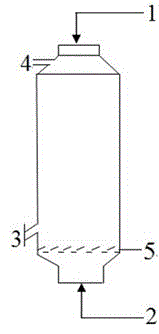
[0038] exist figure 1 In the ebullating bed reactor shown, 30.0 g of vanadium pentoxide; 690 mL of mixed solution of isobutanol and benzyl alcohol is added through feed port 1, and the volume ratio of isobutanol / benzyl alcohol is 10:1. Will N 2 It is blown into the reactor from the feed port 2 to keep the reaction liquid and solid particles in a "boiling" tumbling state. Raise the reaction temperature and keep it at 110±2°C, and keep the reaction time for 3 hours; then feed 38.0 g of concentrated phosphoric acid with a concentration of 85% into the reactor from feed port 1, and the phosphorus / vanadium molar ratio is 1.0. Continue to keep the "boiling" reaction for 6 hours, and the reaction is completed and discharged from the reaction material outlet 3. After the reaction solution was cooled to room temperature, it was filtered, and the filter cake was rinsed three times with a small amount of isobutanol, and then the filter cake was placed in an enamel dish and dried natura...
Embodiment 2
[0040] exist figure 1 In the ebullating bed reactor shown, add 30g of vanadium pentoxide, 0.5g of zirconium nitrate as auxiliary agent, 0.95g of nickel nitrate hexahydrate as auxiliary agent; 690mL of isobutanol and benzyl alcohol mixed solution, The volume ratio of benzyl alcohol is 15:1. Will N 2 It is blown into the reactor from the feed port 2 to keep the reaction liquid and solid particles in a "boiling" tumbling state. Raise the reaction temperature and keep it at 100±2°C, and keep the reaction time for 4 hours; then feed 35.5g of concentrated phosphoric acid with a concentration of 100% into the reactor from the feed port 1, and the phosphorus / vanadium molar ratio is 1.1, and continue to maintain " Boiling shape" was reacted for 6 hours, and the reaction was completed and discharged from the reaction material outlet 3. After the reaction solution was cooled to room temperature, it was filtered, and the filter cake was rinsed three times with a small amount of isobuta...
Embodiment 3
[0042] exist figure 1 In the ebullating bed reactor shown, 30.0 g of vanadium pentoxide; 690 mL of mixed solution of isobutanol and benzyl alcohol is added through feed port 1, and the volume ratio of isobutanol / benzyl alcohol is 20:1. Will N 2 It is blown into the reactor from the feed port 2 to keep the reaction liquid and solid particles in a "boiling" tumbling state. Raise the reaction temperature and keep it at 98±2°C, and keep the reaction time for 4 hours; then feed 34.0 g of concentrated phosphoric acid with a concentration of 95% into the reactor from feed port 1, and the phosphorus / vanadium molar ratio is 1.0. Continue to keep the "boiling" reaction for 8 hours, and the reaction is completed and discharged from the reaction material outlet 3. After the reaction solution was cooled to room temperature, it was filtered, and the filter cake was rinsed three times with a small amount of isobutanol, and then the filter cake was placed in an enamel tray and dried natural...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com