Basic antimicrobial peptides and their targeted design and application
An antimicrobial peptide and targeting technology, applied in antibacterial drugs, antifungal agents, peptides, etc., can solve the problems of hemolytic toxicity, lack of targeted antibacterial and anticancer properties, antibiotic resistance, etc., and achieve strong anticancer activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Minimum Inhibitory Concentration (MIC) Determination
[0045] According to the polypeptide sequence in Table 1, various polypeptides were synthesized by fmoc solid-phase synthesis by Shanghai Botai Biotechnology Co., Ltd., and purified by RP-HPLC; the polypeptides include antimicrobial peptides and targeted antimicrobial peptides, wherein the targeted antimicrobial peptides are It is obtained by linking the antimicrobial peptide and the complementarity determining region of the antibody through several leucines.
[0046] The bacteria used were Escherichia coli (MG1655), Pseudomonas aeruginosa (1.2464, Beijing China General Microorganism Culture Collection Management Center), Staphylococcus aureus (ATCC6538), multi-drug resistant Staphylococcus aureus Y5 (Zhang Ying et al., together with food Molecular typing of Staphylococcus aureus in poisoning events, Chinese Journal of Preventive Medicine, 2008, 42(9):672-676; RanHe et al. A combinatorial yeast overlay m...
Embodiment 2
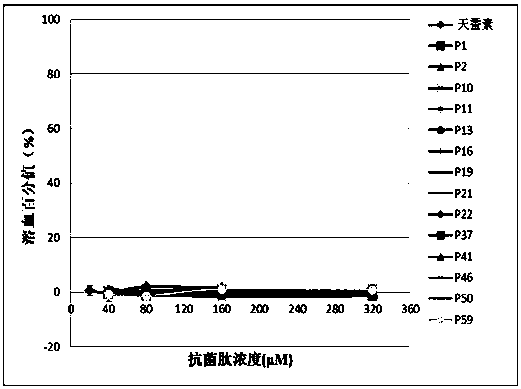
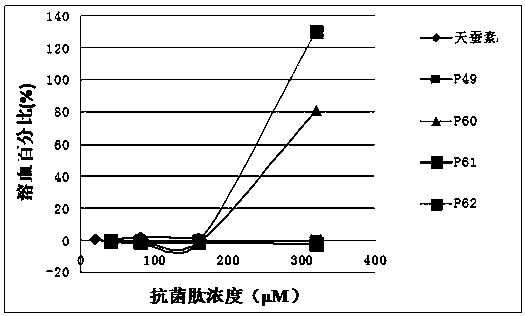
[0074] Embodiment 2 hemolytic experiment
[0075] 1. Blood Processing
[0076] (1) Collect 5 ml of fresh human blood and put it into a tube containing 0.5 ml of 3.8% sodium citrate, blow and beat the blood in the anticoagulant tube to make it fully mix with the anticoagulant.
[0077] (2) The above blood was centrifuged at 2000 rpm for 8 minutes, and the supernatant was removed. Rinse the blood with 10 mM PBS, pH=7.4, centrifuge at 2000 rpm for 5 minutes, discard the supernatant, and repeat the operation until the erythrocyte suspension is clear and free of impurities such as serum, then discard the supernatant. Then it was dissolved in PBS at 5% (v / v) to obtain erythrocyte suspension.
[0078] 2. Antimicrobial peptide treatment: the antimicrobial peptide was dissolved in PBS to obtain antimicrobial peptide solutions with final concentrations of 40 μM, 80 μM, 160 μM, and 320 μM.
[0079] 3. System mixing
[0080] Draw 50 microliters of the treated antimicrobial peptide sol...
Embodiment 3
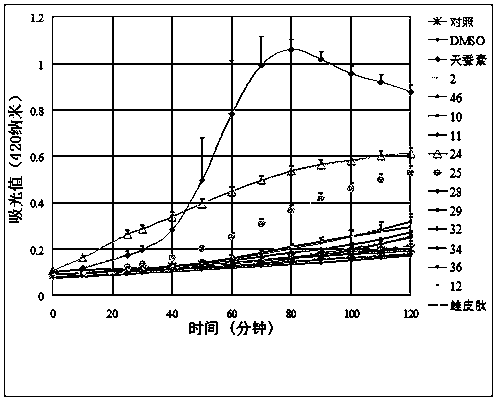
[0086] Example 3 Escherichia coli membrane permeability experiment
[0087]β-galactosidase is a hydrolytic enzyme located in the cytoplasm of bacteria that can hydrolyze o-nitrophenyl β-D-galactopyranoside (ONPG) into galactose and o-nitrophenol (yellow). Add a certain amount of ONPG to the system, and measure the change of the absorbance value of the culture medium at 420 nanometers to determine the degree of hydrolysis of ONPG, so as to determine whether β-galactosidase hydrolyzes ONPG. Generally, ONPG cannot enter the cell because the enzyme is located inside the cell. But once the permeability of the cell membrane changes, ONPG enters the cell and is hydrolyzed, and the culture medium quickly turns yellow, A 420 The value rises rapidly in a short period of time. Therefore, this method can be used to detect the effect of antimicrobial peptides on cell membrane permeability. High membrane permeability indicates that antimicrobial peptides can form membrane channels.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com