Antibacterial peptide of bactrocera dorsalis hendel and derivatives and application of antibacterial peptide
A technology of Bactrocera dorsalis and antimicrobial peptides, applied in the fields of Bacteralis dorsalis antimicrobial peptides and their derivatives and applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
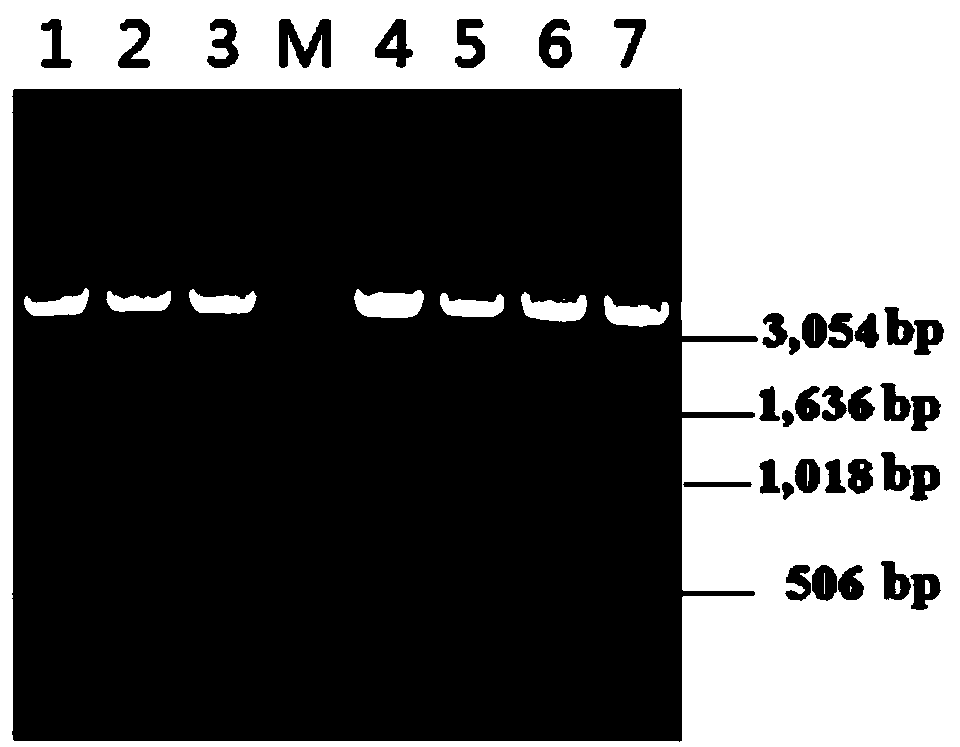
[0031] Example 1 Construction of cDNA library and EST sequence analysis of Bactrocera dorsalis larvae induced by mixed bacteria
[0032] Staphylococcus aureus and Escherichia coli K 12 D. 31 The larvae of Bactrocera dorsalis were needled with mixed bacteria, and after 24 hours of induction, the total RNA of Bactrocera dorsalis larvae was extracted according to the instruction manual of the invitrogen TRIzol Reagent RNA extraction kit. It was observed that the characteristic bands of 18S and 28S were neat and clear, and the brightness ratio was close to 1:2, while measuring OD by UV spectrophotometer 260 / OD 280 =1.82, indicating that the quality of total RNA is better. The library was constructed using the Smart cDNA Library Construction Kit kit from Clontech Company, and experiments were carried out using the primers, vectors, and restriction endonucleases attached to the kit itself. Get the total RNA of 1 μg Bacteralis dorsalis larvae, with SMART III oligonucleotide frag...
Embodiment 2
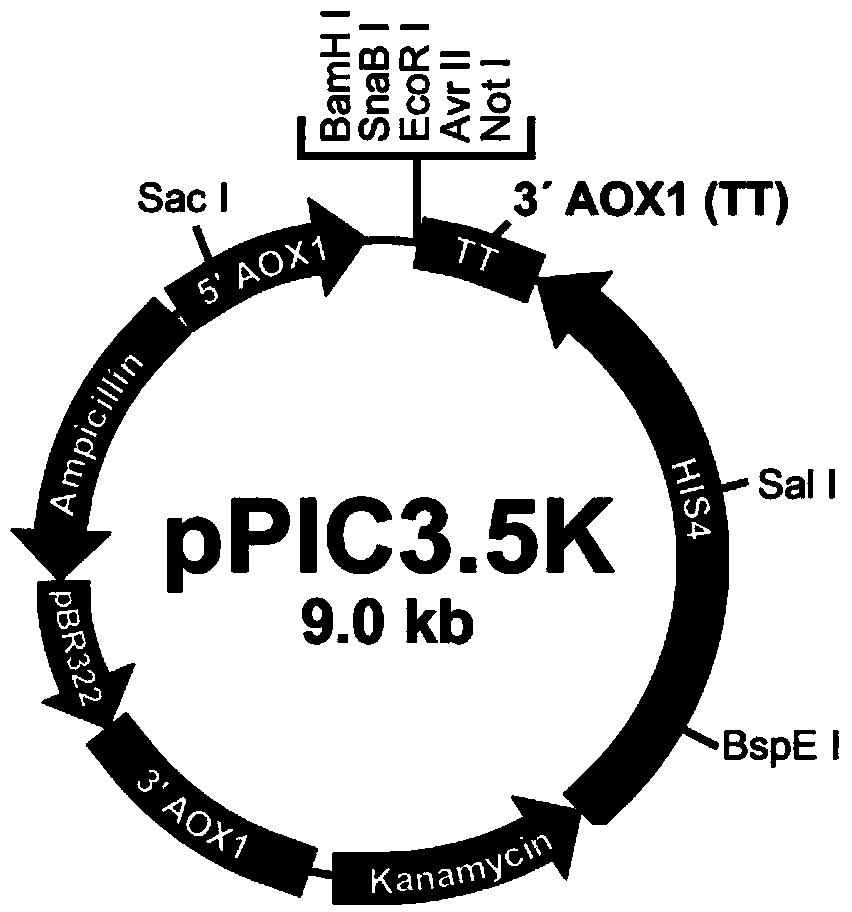
[0035] Example 2 Recombinant Expression of Bacteralis dorsalis Antimicrobial Peptide BH-AMP003
[0036] In this experiment, the histidine-deficient Pichia pastoris GS115 was used as the host strain, and the intracellular expression vector pPIC3.5K was used to construct the recombinant expression system. Select the Sac I endonuclease to linearize the recombinant vector pPIC3.5K, and then pass the BH-AMP003 gene (shown in SEQ ID NO: 3) with the corresponding restriction site through molecular cloning T4 DNA ligase was ligated with the linearized vector, and then transformed into Escherichia coli DH5α, and positive transformants were screened using ampicillin-resistant LB plates. Pick a single colony for inoculation and culture, and extract the plasmid for enzyme digestion verification and sequencing. For the detailed method of the whole experiment, please refer to "Molecular Cloning Laboratory Manual" edited by Sam brook J. The recombinant vector was further transformed into P...
Embodiment 3
[0063] Example 3 Mouse Acute Toxicity Test of Bacteralis dorsalis Antimicrobial Peptide BH-AMP003 Expressed by Recombinant Yeast
[0064] The recombinant yeast expressing the Bacteralis dorsalis antimicrobial peptide BH-AMP003 prepared above was fermented, and the fermented liquid was spray-dried to obtain a dry bacterial liquid powder of the Bacteralis dorsalis antibacterial peptide BH-AMP003, which was used in subsequent experiments.
[0065] Select 40 healthy NIH mice, male or female, weighing about 30 g, and randomly divide them into two groups, namely, the blank control group and the BH-AMP003 bacteria solution dry powder group, with 20 mice in each group. Before the experiment, the weight of the mice was recorded once. At the beginning of the experiment, the mice in each group were given the test sample at a time with a maximum administration volume of 0.8ml. After the administration, the mice were continuously observed for 7 days to observe whether the mice appeared ment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com