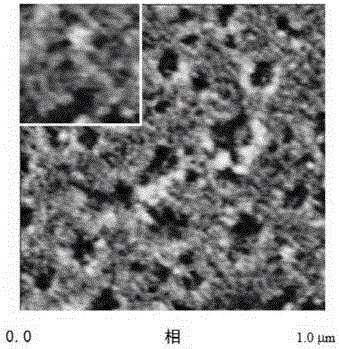
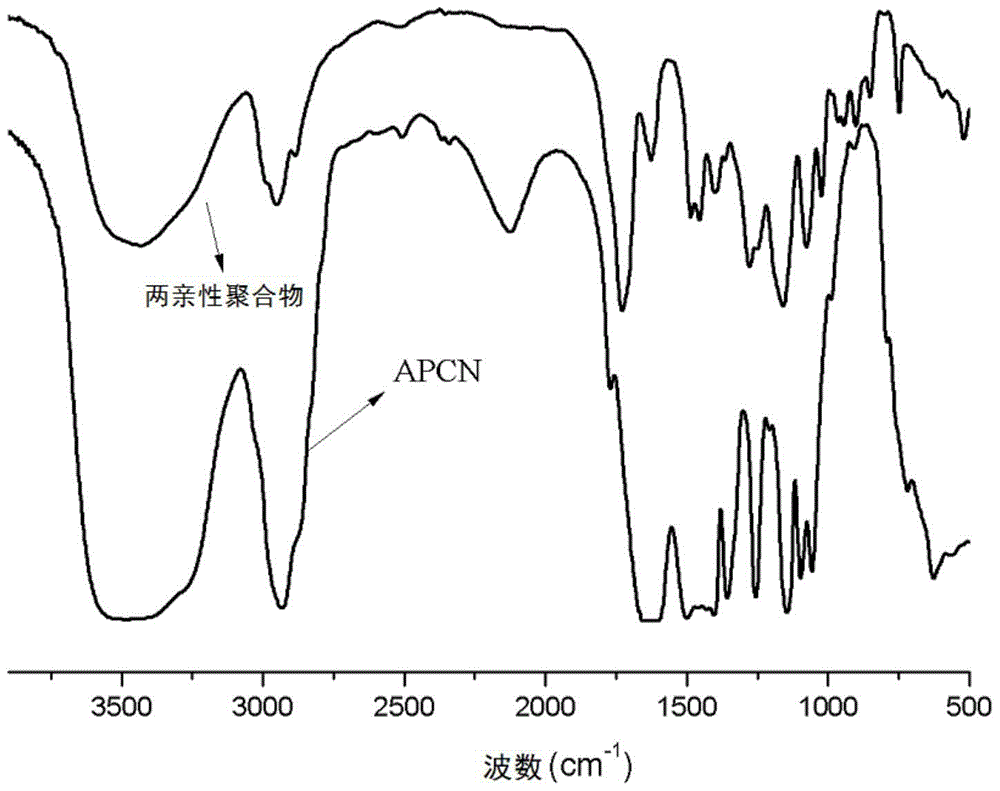
A kind of amphiphilic copolymer network and preparation method thereof
An amphiphilic copolymer and copolymer technology, applied in the field of medical polymer materials, can solve the problems of uncontrollable network structure size, poor mechanical properties of network structure, poor controllability of molecular weight, etc., and achieve easy molecular design and good swelling performance , Good cross-linking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] In the method for preparing the amphiphilic copolymer network of the present invention, preferably the triethanolamine in the first step is refined triethanolamine.
[0045] Preferably, the acid-binding agent described in the first step is triethylamine, sodium hydroxide, potassium hydroxide or pyridine.
[0046] Preferably, the nucleophilic substitution reagent described in the first step is bromoacyl bromide or chloroacyl chloride.
[0047] Preferably, the hydrophilic monomer described in the second step is an acrylamide monomer, an acrylamide monomer, an acrylate monomer or a methacrylate monomer.
[0048] Preferably, the ligand A described in the second step is 2'2-bipyridine (Bpy), tris-(N,N-dimethylaminoethyl)amine (Me 6 TREN), pentamethyldiethylenetriamine (PDMAETA), and 4-dimethylaminopyridine (DMAP) or a mixture of two or more.
[0049] Preferably, the first catalyst described in the second step is cuprous chloride, cuprous bromide or ferrous chloride.
[00...
Embodiment 1
[0078] (1) Triethanolamine is distilled under reduced pressure to obtain refined triethanolamine, 10 parts of refined triethanolamine are dissolved in 600 parts of tetrahydrofuran THF to obtain triethanolamine solution, 80 parts of triethylamine are added, and 30 parts of 2-bromoisobutyl are added dropwise Acyl bromide was reacted in an ice-water bath for 3 hours. After removing the ice-water bath, it was reacted at room temperature for 6 hours. The product was filtered to remove the white precipitate, and the solvent was removed, then dissolved in n-hexane, washed with deionized water, and dried for 24 hours to obtain the terminal band Br's star ATRP small molecule initiator.
[0079] (2) 0.8 parts of 2'2-bipyridine, 0.48 parts of star ATRP small molecule initiator, 16 parts of hydroxyethyl methacrylate (HEMA), 0.0768 parts of cuprous chloride, 14 parts of n-propanol, butanone 6 parts were mixed, after deoxygenation at -20°C, reacted at 5°C for 24 hours, passed the mixture th...
Embodiment 2
[0091] (1) Triethanolamine is distilled under reduced pressure to obtain refined triethanolamine, 10 parts of refined triethanolamine are dissolved in 800 parts of dichloromethane to obtain triethanolamine solution, 70 parts of sodium hydroxide are added, and 70 parts of 2-bromo Isobutyryl bromide was reacted in an ice-water bath for 3 hours. After removing the ice-water bath, it was reacted at room temperature for 6 hours. The product was filtered to remove the white precipitate, and the solvent was removed, then dissolved in n-hexane, washed with deionized water, and dried for 24 hours to obtain Star-shaped ATRP small molecule initiator with Br at the end.
[0092] (2) 0.8 parts of 2'2-bipyridine, 0.64 parts of star ATRP small molecule initiator, 20 parts of dimethylaminoethyl methacrylate (DMAEA), 0.2 parts of cuprous chloride, 21 parts of n-propanol, Mix 9 parts of butanone, remove oxygen at -20°C, react at 50°C for 24 hours, pass the mixture through a neutral alumina chro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com